Dev Rev. 2008; 28(1): 62–77. doi: 10.1016/j.dr.2007.08.003
Abstract
Adolescence is a developmental period characterized by suboptimal decisions and actions that give rise to an increased incidence of unintentional injuries and violence, alcohol and drug abuse, unintended pregnancy and sexually transmitted diseases. Traditional neurobiological and cognitive explanations for adolescent behavior have failed to account for the nonlinear changes in behavior observed during adolescence, relative to childhood and adulthood. This review provides a biologically plausible conceptualization of the neural mechanisms underlying these nonlinear changes in behavior, as a heightened responsiveness to incentives while impulse control is still relatively immature during this period. Recent human imaging and animal studies provide a biological basis for this view, suggesting differential development of limbic reward systems relative to top-down control systems during adolescence relative to childhood and adulthood. This developmental pattern may be exacerbated in those adolescents with a predisposition toward risk-taking, increasing the risk for poor outcomes.
According to the National Center for Health Statistics, there are over 13,000 adolescent deaths in the United States each year. Approximately 70% of these deaths result from motor vehicle crashes, unintentional injuries, homicide, and suicide (Eaton et al., 2006). Results from the 2005 National Youth Risk Behavior Survey (YRBS) show that adolescents engage in behaviors that increase their likelihood of death or illness by driving a vehicle after drinking or without a seat belt, carrying weapons, using illegal substances, and engaging in unprotected sex resulting in unintended pregnancies and STDs, including HIV infection (Eaton et al., 2006). These statistics underscore the significance of understanding risky choices and actions in adolescents.
A number of cognitive and neurobiological hypotheses have been postulated for why adolescents engage in suboptimal choice behavior. In a recent review of the literature on human adolescent brain development, Yurgelun-Todd (2007) suggests that cognitive development through the adolescent years is associated with progressively greater efficiency of cognitive control capacities. This efficiency is described as dependent on maturation of the prefrontal cortex as evidenced by increased activity within focal prefrontal regions (Rubia et al., 2000; Tamm, Menon, & Reiss, 2002) and diminished activity in irrelevant brain regions (Brown et al., 2005; Durston et al., 2006).
This general pattern, of improved cognitive control with maturation of the prefrontal cortex, suggests a linear increase in development from childhood to adulthood. Yet suboptimal choices and actions observed during adolescence represent a nonlinear change in behavior that can be distinguished from childhood and adulthood, as evidenced by the National Center for Health Statistics on adolescent behavior and mortality. If cognitive control and an immature prefrontal cortex were the basis for suboptimal choice behavior, then children should look remarkably similar or even worse than adolescents, given their less developed prefrontal cortex and cognitive abilities. Thus, immature prefrontal function alone, cannot account for adolescent behavior.
An accurate conceptualization of cognitive and neurobiological changes during adolescence must treat adolescence as a transitional developmental period (Spear, 2000), rather than a single snapshot in time (Casey, Tottenham, Liston, & Durston, 2005). In other words, to understand this developmental period, transitions into and out of adolescence are necessary for distinguishing distinct attributes of this stage of development. Establishing developmental trajectories for cognitive and neural processes is essential in characterizing these transitions and constraining interpretations about changes in behavior during this period. On a cognitive or behavioral level, adolescents are characterized as impulsive (i.e., lacking cognitive control) and risk-taking with these constructs used synonymously and without appreciation for distinct developmental trajectories of each. On a neurobiological level, human imaging and animal studies suggest distinct neurobiological bases and developmental trajectories for the neural systems that underlie these separate constructs of impulse control and risky decisions.
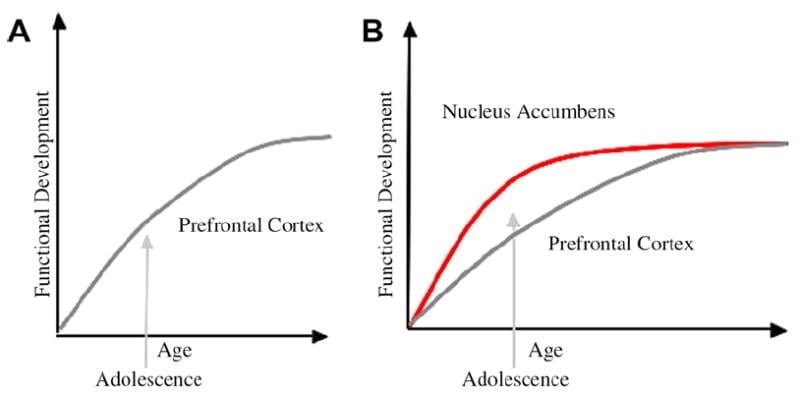
We have developed a neurobiological model of adolescent development within this framework that builds on rodent models (Laviola, Adriani, Terranova, & Gerra, 1999; Spear, 2000) and recent imaging studies of adolescence (Ernst et al., 2005; Galvan, Hare, Voss, Glover, & Casey, 2007; Galvan et al., 2006). Fig. 1 below depicts this model. On the left is the traditional characterization of adolescence as related almost exclusively to the immaturity of the prefrontal cortex. On the right is our proposed neurobiological model that illustrates how limbic subcortical and prefrontal top-down control regions must be considered together. The cartoon illustrates different developmental trajectories for these systems, with limbic systems developing earlier than prefrontal control regions. According to this model, the individual is biased more by functionally mature limbic regions during adolescence (i.e., imbalance of limbic relative to prefrontal control), compared to children, for whom these systems (i.e., limbic and prefrontal) are both still developing; and compared to adults, for whom these systems are fully mature. This perspective provides a basis for nonlinear shifts in behavior across development, due to earlier maturation of this limbic relative to less mature top-down prefrontal control region. With development and experience, the functional connectivity between these regions provides a mechanism for top-down control of these regions (Hare, Voss, Glover, & Casey, 2007a). Further, the model reconciles the contradiction of health statistics of risky behavior during adolescence, with the astute observation by Reyna and Farley (2006) that adolescents are able to reason and understand risks of behaviors in which they engage. According to our model, in emotionally salient situations, the limbic system will win over control systems given its maturity relative to the prefrontal control system. Evidence from behavioral and human imaging studies to support this model are provided in the context of actions in rewarding and emotional contexts (Galvan et al., 2006, 2007; Hare, Voss, Glover, & Casey, 2007b; Hare et al., 2007a). In addition, we speculate on why the brain may develop in this way and why some teenagers may be at greater risk for making suboptimal decisions leading to poorer long-term outcomes (Galvan et al., 2007; Hare et al., 2007b).
The traditional explanation of adolescent behavior has been suggested to be due to the protracted development of the prefrontal cortex (A). Our model takes into consideration the development of the prefrontal cortex together with subcortical limbic regions (e.g., nucleus accumbens) that have been implicated in risky choices and actions (B).
Development of goal-directed behavior
A cornerstone of cognitive development is the ability to suppress inappropriate thoughts and actions in favor of goal-directed ones, especially in the presence of compelling incentives (Casey, Galvan, & Hare, 2005; Casey et al., 2000b; Casey, Thomas, David-son, Kunz, & Franzen, 2002a; Casey, Tottenham, & Fossella, 2002b). A number of classic developmental studies have shown that this ability develops throughout childhood and adolescence (Case, 1972; Flavell, Feach, & Chinsky, 1966; Keating & Bobbitt, 1978; Pascual-Leone, 1970). Several theorists have argued that cognitive development is due to increases in processing speed and efficiency and not due to an increase in mental capacity (e.g., Bjorkland, 1985; Bjorkland, 1987; Case, 1985). Other theorists have included the construct of “inhibitory” processes in their account of cognitive development (Harnishfeger & Bjorkland, 1993). According to this account, immature cognition is characterized by susceptibility to interference from competing sources that must be suppressed (e.g., Brainerd & Reyna, 1993; Casey, Thomas, Davidson, Kunz, & Franzen, 2002a; Dempster, 1993; Diamond, 1985; Munakata & Yerys, 2001). Thus goal-directed behavior requires the control of impulses or delay of gratification for optimization of outcomes and this ability appears to mature across childhood and adolescence.
Adolescent behavior has been described as impulsive and risky, almost synonymously, yet these constructs rely on different cognitive and neural processes, that suggest distinct constructs with different developmental trajectories. Specifically, a review of the literature suggests that impulsivity diminishes with age across childhood and adolescence (Casey et al., 2002a; Casey, Galvan et al., 2005; Galvan et al., 2007) and is associated with protracted development of the prefrontal cortex (Casey, Galvan et al., 2005), although there are differences in the degree to which a given individual is impulsive or not, regardless of age.
In contrast, to impulse/cognitive control, risk-taking appears to increase during adolescence relative to childhood and adulthood and is associated with subcortical systems known to be involved in evaluation of rewards. Human imaging studies that will be reviewed, suggest an increase in subcortical activation (e.g., accumbens) when making risky choices (Kuhnen & Knutson, 2005; Matthews & et al., 2004; Montague & Berns, 2002) that is exaggerated in adolescents, relative to children and adults (Ernst et al., 2005; Galvan et al., 2006). These findings suggest different trajectories for reward- or incentive-based behavior, with earlier development of these systems relative to control systems that show a protracted and linear developmental course, in terms of overriding inappropriate choices and actions in favor of goal-directed ones.
Evidence from neuroimaging studies of human brain development
Recent investigations of adolescent brain development have been based on advances in neuroimaging methodologies that can be easily used with developing human populations. These methods rely on magnetic resonance imaging (MRI) methods (see Fig. 2) and include: structural MRI, which is used to measure the size and shape of structures; functional MRI which is used to measure patterns of brain activity; and diffusion tensor imaging (DTI) which is used to index connectivity of white matter fiber tracts. Evidence for our developmental model of competition between cortical and subcortical regions is supported by immature structural and functional connectivity as measured by DTI and fMRI, respectively.
MRI studies of human brain development
Several studies have used structural MRI to map the anatomical course of normal brain development (see review by Durston et al., 2001). Although total brain size is approximately 90% of its adult size by age six, the gray and white matter subcomponents of the brain continue to undergo dynamic changes throughout adolescence. Data from recent longitudinal MRI studies indicate that gray matter volume has an inverted U-shape pattern, with greater regional variation than white matter (Giedd, 2004; Gogtay et al., 2004; Sowell et al, 2003; Sowell, Thompson, & Toga, 2004). In general, regions subserving primary functions, such as motor and sensory systems, mature earliest; higher-order association areas, which integrate these primary functions, mature later (Gogtay et al., 2004; Sowell, Thompson, & Toga, 2004). For example, studies using MRI-based measures show that cortical gray matter loss occurs earliest in the primary sensorimotor areas and latest in the dorsolateral prefrontal and lateral temporal cortices (Gogtay et al., 2004). This pattern is consistent with nonhuman primate and human postmortem studies showing that the prefrontal cortex is one of the last brain regions to mature (Bourgeois, Goldman-Rakic, & Rakic, 1994; Huttenlocher, 1979). In contrast to gray matter, white matter volume increases in a roughly linear pattern, increasing throughout development well into adulthood (Gogtay et al., 2004). These changes presumably reflect ongoing myelination of axons by oligodendrocytes enhancing neuronal conduction and communication.
Although less attention has been given to subcortical regions when examining structural changes, some of the largest changes in the brain across development are seen in these regions, particular in the basal ganglia (Sowell et al., 1999, see Fig. 3) and especially in males (Giedd et al., 1996). Developmental changes in structural volume within basal ganglia and prefrontal regions are interesting in light of known developmental processes (e.g., dendritic arborization, cell death, synaptic pruning, myelination) that are occurring during childhood and adolescence. These processes allow for fine tuning and strengthening of connections between prefrontal and subcortical regions with development and learning that may coincide with greater cognitive control. How do these structural changes relate to cognitive changes? A number of studies have related frontal lobe structural maturation and cognitive function using neuropsychological and cognitive measures (e.g., Sowell et al., 2003). Specifically, associations have been reported between MRI-based prefrontal cortical and basal ganglia regional volumes and measures of cognitive control (i.e., ability to override an inappropriate response in favor of another or to suppress attention toward irrelevant stimulus attribute in favor of relevant stimulus attribute (Casey, Trainor et al., 1997). These findings suggest that cognitive changes are reflected in structural brain changes and underscore the importance of subcortical (basal ganglia) as well as cortical (e.g., prefrontal cortex) development.

DTI studies of human brain development
The MRI-based morphometry studies reviewed suggest that cortical connections are being fine-tuned with the elimination of an overabundance of synapses and strengthening of relevant connections with development and experience. Recent advances in MRI technology, like DTI provide a potential tool for examining the role of specific white matter tracts to the development of the brain and behavior with greater detail. Relevant to this paper are the neuroimaging studies that have linked the development of fiber tracts with improvements in cognitive ability. Specifically, associations between DTI-based measures of prefrontal white matter development and cognitive control in children have been shown. In one study, development of this capacity was positively correlated with prefrontal-parietal fiber tracts (Nagy, Westerberg, & Klingberg, 2004) consistent with functional neuroimaging studies showing differential recruitment of these regions in children relative to adults.
Using a similar approach, Liston et al. (2005) have shown that white matter tracts between prefrontal-basal ganglia and -posterior fiber tracts continue to develop across childhood into adulthood, but only those tracts between the prefrontal cortex and basal ganglia are correlated with impulse control, as measured by performance on a go/nogo task. The prefrontal fiber tracts were defined by regions of interests identified in a fMRI study using the same task. Across both developmental DTI studies, fiber tract measures were correlated with development, but specificity of particular fiber tracts with cognitive performance were shown by dissociating the particular tract (Liston et al., 2005) or cognitive ability (Nagy et al., 2004). These findings underscore the importance of examining not only regional, but circuitry related changes when making claims about age-dependent changes in neural substrates of cognitive development.
Functional MRI studies of behavioral and brain development
Although structural changes measured by MRI and DTI have been associated with behavioral changes during development, a more direct approach for examining structure–function association is to measure changes in the brain and behavior simultaneously, as with fMRI. The ability to measure functional changes in the developing brain with MRI has significant potential for the field of developmental science. In the context of the current paper, fMRI provides a means for constraining interpretations of adolescent behavior. As stated previously, the development of the prefrontal cortex is believed to play an important role in the maturation of higher cognitive abilities such as decision-making and cognitive control (Casey, Tottenham, & Fossella 2002b; Casey, Trainor et al., 1997). Many paradigms have been used, together with fMRI, to assess the neurobiological basis of these abilities, including flanker, Stroop and go/nogo tasks (Casey, Castellanos et al., 1997; Casey, Giedd, & Thomas, 2000a; Durston et al., 2003). Collectively, these studies show that children recruit distinct but often larger, more diffuse prefrontal regions when performing these tasks than do adults. The pattern of activity within brain regions central to task performance (i.e., that correlate with cognitive performance) become more focal or fine-tuned with age, while regions not correlated with task performance diminish in activity with age. This pattern has been observed across both cross-sectional (Brown et al., 2005) and longitudinal studies (Durston et al., 2006) and across a variety of paradigms. Although neuroimaging studies cannot definitively characterize the mechanism of such developmental changes (e.g., dendritic arborization, synaptic pruning) the findings reflect development within, and refinement of, projections to and from, activated brain regions with maturation and suggest that these changes occur over a protracted period of time (Brown et al., 2005; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Casey, Trainor et al., 1997; Casey et al., 2002a; Crone, Donohue, Honomichl, Wendelken, & Bunge, 2006; Luna et al., 2001; Moses et al., 2002; Schlaggar et al., 2002; Tamm et al., 2002; Thomas et al., 2004; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003).
How can this methodology inform us about whether adolescents are indeed lacking sufficient cognitive control (impulsive) or are risky in their choices and actions? Impulse control as measured by cognitive control tasks like the go/nogo task show a linear pattern of development across childhood and adolescence as described above. However, recent neuroimaging studies have begun to examine reward-related processing specific to risk-taking in adolescents (Bjork et al., 2004; Ernst et al., 2005; May et al., 2004). These studies have focused primarily on the region of the accumbens, a portion of the basal ganglia involved in predicting reward, rather than characterization of the development of this region in conjunction with top-down control regions (prefrontal cortex). Although a recent report of less ventral prefrontal activity in adolescents relative to adults during a monetary decision-making task on risk-taking behavior has been shown (Eshel, Nelson, Blair, Pine, & Ernst, 2007).
Overall, few studies have examined how the development of reward circuitry in subcortical regions (e.g., accumbens) changes in conjunction with development of cortical prefrontal regions. Moreover, how these neural changes coincide with reward-seeking, impulsivity and risk-taking behaviors remains relatively unknown. Our neurobiological model proposes that the combination of heightened responsiveness to rewards and immaturity in behavioral control areas may bias adolescents to seek immediate, rather than long-term gains, perhaps explaining their increase in risky decision-making and impulsive behaviors. Tracking subcortical (e.g., accumbens) and cortical (e.g., prefrontal) development of decision-making across childhood through adulthood, provides additional constraints on whether changes reported in adolescence are specific to this period of development, or reflect maturation that is steadily occurring in a somewhat linear pattern from childhood to adulthood.
Empirical evidence from a recent fMRI study helps to support our neurobiological model and takes a transitional approach to understanding adolescence by examining changes prior to and following adolescence. In this study (Galvan et al., 2006), we examined behavioral and neural responses to reward manipulations across development, focusing on brain regions implicated in reward-related learning and behavior in animal (Hikosaka & Watanabe, 2000; Pecina, Cagniard, Berridge, Aldridge, & Zhuang, 2003; Schultz, 2006) and adult imaging studies (e.g., Knutson, Adams, Fong, & Hommer, 2001; O,Doherty, Kringelbach, Rolls, Hornak, Andrews, 2001; Zald et al., 2004) and in studies of addiction (Hyman & Malenka, 2001; Volkow & Li, 2004). Based on rodent models (Laviola et al., 1999; Spear, 2000) and previous imaging work (Ernst et al., 2005), we hypothesized that relative to children and adults, adolescents would show exaggerated activation of the accumbens, in concert with less mature recruitment of top-down prefrontal control regions. Recent work showing delayed functional connectivity between these prefrontal and limbic subcortical regions in adolescence relative to adults, provides a mechanism for the lack of top-down control of these regions (Hare et al., 2007a).
Our findings were consistent with rodent models (Laviola, Macri, Morley-Fletcher, & Adriani, 2003) and previous imaging studies (Ernst et al., 2005) suggesting enhanced accumbens activity to rewards during adolescence. Indeed, relative to children and adults, adolescents showed an exaggerated accumbens response in anticipation of reward. However, both children and adolescents showed a less mature response in prefrontal control regions than adults. These findings suggest different developmental trajectories for these regions may underlie the enhancement in accumbens activity, relative to children or adults, which may in turn relate to the increased impulsive and risky behaviors observed during this period of development (see Fig. 4).

Differential recruitment of prefrontal and subcortical regions has been reported across a number of developmental fMRI studies (Casey et al., 2002b; Monk et al., 2003; Thomas et al., 2004). Typically these findings have been interpreted in terms of immature prefrontal regions rather than an imbalance between prefrontal and subcortical regional development. Given evidence of prefrontal regions in guiding appropriate actions in different contexts (Miller & Cohen, 2001) immature prefrontal activity might hinder appropriate estimation of future outcomes and appraisal of risky choices, and might thus be less influential on reward valuation than the accumbens. This pattern is consistent with previous research showing elevated subcortical, relative to cortical activity when decisions are biased by immediate over long-term gains (McClure, Laibson, Loewenstein, & Cohen, 2004). Further, accumbens activity has been shown with fMRI to positively correlate with subsequent risk-taking behaviors (Kuhnen & Knutson, 2005). During adolescence, relative to childhood or adulthood, immature ventral prefrontal cortex may not provide sufficient top-down control of robustly activated reward processing regions (e.g., accumbens), resulting in less influence of prefrontal systems (orbitofrontal cortex) relative to the accumbens in reward valuation.
Why would the brain be programmed to develop this way?
Adolescence is the transitional period between childhood and adulthood often co-occurring with puberty. Puberty marks the beginnings of sexual maturation (Graber & Brooks-Gunn, 1998) and can be defined by biological markers. Adolescence can be described as a progressive transition into adulthood with a nebulous ontogenetic time course (Spear, 2000). Evolutionarily speaking, adolescence is the period in which independence skills are acquired to increase success upon separation from the protection of the family, though increase chances for harmful circumstances (e.g., injury, depression, anxiety, drug use and addiction (Kelley, Schochet, & Landry, 2004). Independence-seeking behaviors are prevalent across species, such as increases in peer-directed social interactions and intensifications in novelty-seeking and risk-taking behaviors. Psychosocial factors impact adolescent propensity for risky behavior. However, risky behavior is the product of a biologically driven imbalance between increased novelty- and sensation-seeking in conjunction with immature “self-regulatory competence” (Steinberg, 2004). Our neurobiological data suggest this occurs through differential development of these two systems (limbic and control).
Speculation would suggest that this developmental pattern is an evolutionary feature. You need to engage in high-risk behavior to leave your family and village to find a mate and risk-taking at just the same time as hormones drive adolescents to seek out sexual partners. In today’s society when adolescence may extend indefinitely, with children living with parents and having financial dependence and choosing mates later in life, this evolution may be deemed inappropriate.
There is evidence across species for heightened novelty-seeking and risk-taking during the adolescent years. Seeking out same-age peers and fighting with parents, which all help get the adolescent away from the home territory for mating is seen in other species including rodents, nonhuman primates and some birds (Spear, 2000). Relative to adults, periadolescent rats show increased novelty-seeking behaviors in a free choice novelty paradigm (Laviola et al., 1999). Neurochemical evidence indicates that the balance in the adolescent brain between cortical and subcortical dopamine systems, begins to shift toward greater cortical dopamine levels during adolescence (Spear, 2000). Similar protracted dopaminergic enervation through adolescence into adulthood has been shown in the nonhuman primate prefrontal cortex as well (Rosenberg & Lewis, 1995). Thus this elevated apparent risk-taking appears to be across species and have important adaptive purposes.
Biological predispositions, development, and risk
Individual differences in impulse control and taking risks has been recognized in psychology for some time (Benthin, Slovic, & Severson, 1993). Perhaps one of the classic examples of individual differences reported in these abilities in the social, cognitive and developmental psychology literature is delay of gratification (Mischel, Shoda, & Rodriguez, 1989). Delay of gratification is typically assessed in 3- to 4-year-old toddlers. The toddler is asked whether they would prefer a small reward (one cookie) or a large reward (two cookies). The child is then told that the experimenter will leave the room in order to prepare for upcoming activities and explains to the child that if she remains in her seat and does not eat a cookie, she will receive the large reward. If the child does nor or cannot wait, she should ring a bell to summon the experimenter and thereby receive the smaller reward. Once it is clear the child understands the task, she is seated at the table with the two rewards and the bell. Distractions in the room are minimized, with no toys, books or pictures. The experimenter returns after 15 min or after the child has rung the bell, eaten the rewards, or shown any signs of distress. Mischel showed that children typically behave in one of two ways: (1) either they ring the bell almost immediately in order to have the cookie, which means they only get one; or (2) they wait and optimize their gains, and receive both cookies. This observation suggests that some individuals are better than others in their ability to control impulses in the face of highly salient incentives and this bias can be detected in early childhood (Mischel et al., 1989) and they appear to remain throughout adolescence and young adulthood (Eigsti et al., 2006).
What might explain individual differences in optimal decision-making and behavior? Some theorists have postulated that dopaminergic mesolimbic circuitry, implicated in reward processing, underlies risky behavior. Individual differences in this circuitry, such as allelic variants in dopamine-related genes, resulting in too little or too much dopamine in subcortical regions, might relate to the propensity to engage in risky behavior (O’Doherty, 2004). The nucleus accumbens has been shown to increase in activity immediately prior to making risky choices on monetary-risk paradigms (Kuhnen & Knutson, 2005; Matthews et al., 2004; Montague & Berns, 2002) and as described previously, adolescents show exaggerated accumbens activity to rewarding outcomes relative to children or adults (Ernst et al., 2005; Galvan et al., 2006). Collectively, these data suggest that adolescents may be more prone to risky choices as a group (Gardener & Steinberg, 2005), but some adolescents will be more prone than others to engage in risky behaviors, putting them at potentially greater risk for negative outcomes. Therefore it is important to consider individual variability when examining complex brain–behavior relationships related to risk-taking and reward processing in developmental populations.
To explore individual differences in risk-taking behavior, Galvan et al. (2007) recently examined the association between activity in reward-related neural circuitry in anticipation of a large monetary reward with personality trait measures of risk-taking and impulsivity in adolescence. Functional magnetic resonance imaging and anonymous self-report rating scales of risky behavior, risk perception and impulsivity were acquired in individuals between the ages of 7 and 29 years. There was a positive association between accumbens activity and the likelihood of engaging in risky behavior across development. This activity varied as a function of individuals’ ratings of anticipated positive or negative consequences of such behavior. Those individuals who perceived risky behaviors as leading to dire consequences, activated the accumbens less to reward. This association was driven largely by the children, with the adults rating the consequences of such behavior as possible. Impulsivity ratings were not associated with accumbens activity, but rather with age. These findings suggest that during adolescence, some individuals may be more prone to engage in risky behaviors due to developmental changes in concert with variability in a given individual’s predisposition to engage in risky behavior, rather than to simple changes in impulsivity (see Fig. 5).

Adolescent behavior has repeatedly been characterized as impulsive and risky (Steinberg, 2004, 2007), yet this review of the imaging literature suggests different neurobiological substrates and different developmental trajectories for these behaviors. Specifically, impulsivity is associated with immature ventral prefrontal development and gradually diminishes from childhood to adulthood (Casey, Galvan et al., 2005). The negative correlation between impulsivity ratings and age in the study by Galvan et al. (2007) further supports this notion. In contrast, risk-taking is associated with an increase in accumbens activity (Kuhnen & Knutson, 2005; Matthews et al., 2004; Montague & Berns, 2002), that is exaggerated in adolescents, relative to children and adults (Ernst et al., 2005; Galvan et al., 2006). Thus adolescent choices and behavior cannot be explained by impulsivity or protracted development of the prefrontal cortex alone, as children would then be predicted to be greater risk takers. The findings provide a neural basis for why some adolescents are at greater risk than others, but further provide a basis for how adolescent behavior is different from children and adults in risk-taking.
Collectively, these data suggest that although adolescents as a group are considered risk takers (Gardener & Steinberg, 2005), some adolescents will be more prone than others to engage in risky behaviors, putting them at potentially greater risk for negative outcomes. These findings underscore the importance of considering individual variability when examining complex brain–behavior relationships related to risk-taking and reward processing in developmental populations. Further, these individual and developmental differences may help explain vulnerability in some individuals to risk-taking associated with substance use, and ultimately, addiction.
Conclusions
Human imaging studies show structural and functional changes in frontostriatal regions (Giedd et al., 1996, 1999; Jernigan et al., 1991; Sowell et al., 1999; for review, Casey, Galvan et al., 2005) that seem to parallel increases in cognitive control and self-regulation (Casey, Trainor et al., 1997; Luna & Sweeney, 2004; Luna et al., 2001; Rubia et al., 2000; Steinberg, 2004; see also Steinberg, 2008, this issue). These changes appear to show a shift in activation of prefrontal regions from diffuse to more focal recruitment over time (Brown et al., 2005; Bunge et al., 2002; Casey, Trainor et al., 1997; Durston et al., 2006; Moses et al., 2002) and elevated recruitment of subcortical regions during adolescence (Casey et al., 2002a; Durston et al., 2006; Luna et al., 2001). Although neuroimaging studies cannot definitively characterize the mechanism of such developmental changes, these changes in volume and structure may reflect development within, and refinement of, projections to and from these brain regions during maturation suggestive of fine-tuning of the system with development.
Taken together, the findings synthesized here indicate that increased risk-taking behavior in adolescence is associated with different developmental trajectories of subcortical pleasure and cortical control regions. These developmental changes can be exacerbated by individual differences in activity of reward systems. Although adolescence has been distinguished as a period characterized by reward-seeking and risk-taking behaviors (Gardener & Steinberg, 2005; Spear, 2000) individual differences in neural responses to reward, predispose some adolescents to take more risks than others, putting them at greater risk for negative outcomes. These findings provide crucial groundwork by synthesizing the various finding related to risk-taking behavior in adolescence and in understanding individual differences and developmental markers for propensities to engage in negative behavior.
Acknowledgments
This work was supported in part by grants from the National Institute of Drug Abuse R01 DA18879 and the National Institute of Mental Health 1P50 MH62196.
References
- Benthin A, Slovic P, Severson H. A psychometric study of adolescent risk perception. Journal of Adolescence. 1993;16:153–168. [PubMed]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: Similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. [PubMed]
- Bjorkland DF. The role of conceptual knowledge in the development of organization in children’s memory. In: Brainerd CJ, Pressley M, editors. Basic processes in memory development: Progress in cognitive development research. New York: Springer-Verlag; 1985. pp. 103–142.
- Bjorkland DF. How age changes in knowledge base contribute to the development of children’s memory: An interpretive review. Developmental Review. 1987;7:93–130.
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. [PubMed]
- Brainerd CJ, Reyna VF. Memory independence and memory interference in cognitive development. Psychological Review. 1993;100:42–67. [PubMed]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–290. [PubMed]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. [PubMed]
- Case R. Validation of a neo-Piagetian capacity construct. Journal of Experimental Child Psychology. 1972;14:287–302.
- Case R. Intellectual development: Birth to adulthood. New York: Academic Press; 1985.
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB. et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. [PubMed]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–244. [PubMed]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000a;54:241–257. [PubMed]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. Journal of Neuroscience. 2002a;22:8647–8652. [PubMed]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, et al. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Science. 2000b;97:8728–8733.
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion and genetic approaches toward a model of cognitive control. Developmental Psychobiology. 2002b;40:237–254. [PubMed]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Science. 2005;9:104–110.
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847.
- Crone E, Donohue S, Honomichl R, Wendelken C, Bunge S. Brain regions mediating flexible rule use during development. Journal of Neuroscience. 2006;26:11239–11247. [PubMed]
- Dempster FN. Resistance to interference: Developmental changes in a basic processing mechanism. In: Howe ML, Pasnak R, editors. Emerging themes in cognitive development Volume 1: Foundations. New York: Springer; 1993. pp. 3–27.
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Development. 1985;56:868–883. [PubMed]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20:2135–2141. [PubMed]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella J, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;1:18–20. [PubMed]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: What have we learned? Journal of American Academy of Child Adolescent Psychiatry. 2001;40:1012–1020.
- Eaton LK, Kinchen S, Ross J, Hawkins J, Harris WA, Lowry R, et al. Youth risk behavior surveillance—United States, 2005, surveillance summaries. Morbidity and Mortality Weekly Report. 2006;55:1–108. [PubMed]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science. 2006;17:478–484. [PubMed]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. [PubMed]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. [PMC free article] [PubMed]
- Flavell JH, Feach DR, Chinsky JM. Spontaneous verbal rehearsal in a memory task as a function of age. Child Development. 1966;37:283–299. [PubMed]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. [PubMed]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10:F8–F14. [PubMed]
- Gardener M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology. 2005;41:625–635. [PubMed]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. [PubMed]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863.
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6:551–560. [PubMed]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. [PMC free article] [PubMed]
- Graber JA, Brooks-Gunn J. Puberty. In: Blechman EA, Brownell KD, editors. Behavioral medicine and women a comprehensive handbook. New York, NY: Guilford Press; 1998. pp. 51–58.
- Hare TA, Voss HU, Glover GH, Casey BJ. The adolescent brain and potential risk for anxiety and depression. 2007a Submitted for publication.
- Hare TA, Voss HU, Glover GH, Casey BJ. Competition between prefrontal and subcortical limbic systems underlie emotional reactivity during adolescence. 2007b Submitted for publication.
- Harnishfeger KK, Bjorkland F. The ontogeny of inhibition mechanisms: A renewed approach to cognitive development. In: Howe ML, Pasnek R, editors. Emerging themes in cognitive development. Vol. 1. New York: Springer-Verlag; 1993. pp. 28–49.
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10:263–271. [PubMed]
- Huttenlocher PR. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Research. 1979;163:195–205. [PubMed]
- Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nature Reviews Neuroscience. 2001;2:695–703.
- Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Archives of General Psychiatry. 1991;48:811–823.
- Keating DP, Bobbitt BL. Individual and developmental differences in cognitive processing components of mental ability. Child Development. 1978;49:155–167.
- Kelley AE, Schochet T, Landry C. Annals of the New York Academy of Sciences. 2004;1021:27–32. [PubMed]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159. [PubMed]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. [PubMed]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience and Biobehavioral Reviews. 1999;23:993–1010. [PubMed]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Abstract risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neuroscience and Biobehavioral Reviews. 2003;27:19–31. [PubMed]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2005;16:553–560. [PubMed]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. [PubMed]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. [PubMed]
- Matthews SC, et al. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport. 2004;15:2123–2127. [PubMed]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. [PubMed]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate monetary rewards. Science. 2004;306:503–507. [PubMed]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202.
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. [PubMed]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. [PubMed]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. [PubMed]
- Moses P, Roe K, Buxton RB, Wong EC, Frank LR, Stiles J. Functional MRI of global and local processing in children. Neuroimage. 2002;16:415–424. [PubMed]
- Munakata Y, Yerys BE. All together now: When dissociations between knowledge and action disappear. Pscychological Science. 2001;12:335–337.
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–1233. [PubMed]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neurosci. 2001;4:95–102. [PubMed]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinions in Neurobiology. 2004;14:769–776.
- Pascual-Leone JA. A mathematical model for transition in Piaget’s developmental stages. Acta Psychologica. 1970;32:301–345.
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. Journal of Neuroscience. 2003;23:9395–9402. [PubMed]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making: Implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7:1–44.
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. The Journal of Comparative Neurology. 1995;358:383–400. [PubMed]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24:13–19. [PubMed]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. [PubMed]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Reviews of Psychology. 2006;57:87–115.
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315.
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861.
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. [PubMed]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. [PubMed]
- Steinberg L. Risk-taking in adolescence: What changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. [PubMed]
- Steinberg L. Risk-taking in adolescence: New perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16:55–59.
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. [PMC free article] [PubMed]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. [PubMed]
- Thomas KM, Hunt RH, Vizueta N, Sommer T, Durston S, Yang Y, et al. Evidence of developmental differences in implicit sequence learning: An FMRI study of children and adults. Journal of Cognitive Neuroscience. 2004;16:1339–1351. [PubMed]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773.
- Volkow ND, Li TK. Drug addiction: The neurobiology of behaviour gone awry. Nature Reviews Neuroscience. 2004;5:963–970.
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17:251–257. [PubMed]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, et al. Dopamine transmission in the human striatum during monetary reward tasks. Journal of Neuroscience. 2004;24:4105–4112. [PubMed]