NEWS AND VIEWS
19 December 2018
Brain circuits of compulsive drug addiction identified
A study in mice identifies a brain adaptation that underlies the compulsive behaviour associated with drug addiction, and which might explain why some drug users behave compulsively whereas others do not.
Drugs of abuse have complex pharmacological effects that trigger many changes in brain function. One of these effects, the direct or indirect activation of neurons that release the neurotransmitter dopamine, is common to all drugs of abuse and has long been assumed to contribute to the development of addiction. Writing in Nature, Pascoli et al.1 report on the neurobiological mechanisms induced by the repeated activation of dopamine neurons that might explain why some drug users seek reward despite facing negative consequences — a type of compulsive behaviour that is a defining feature of human addiction2.
The authors took an optogenetics approach to mimic the activation of the brain’s dopamine systems by drugs of abuse: they used laser light delivered through an optical fibre to activate dopamine neurons in the ventral tegmental area (VTA) of the brains of genetically engineered mice. The mice could directly stimulate these neurons themselves by pressing a lever, and performed this action avidly during a test period of 40 minutes a day for almost 2 weeks.
On subsequent days, the mice received a brief electric shock to their feet on one-third of the lever-pressing occasions, at random. Their behaviour under this condition revealed an intriguing variability: 40% of the mice (termed renouncers) greatly reduced the frequency of lever-pressing when given foot shocks (Fig. 1a), whereas the remaining 60% (perseverers) were willing to receive painful punishment for the opportunity to self-stimulate their dopamine neurons (Fig. 1b). As some of these authors have previously shown3, the persevering mice provide a model for persistent drug use despite negative consequences, and parallel the subset of human drug users whose drug use becomes compulsive.
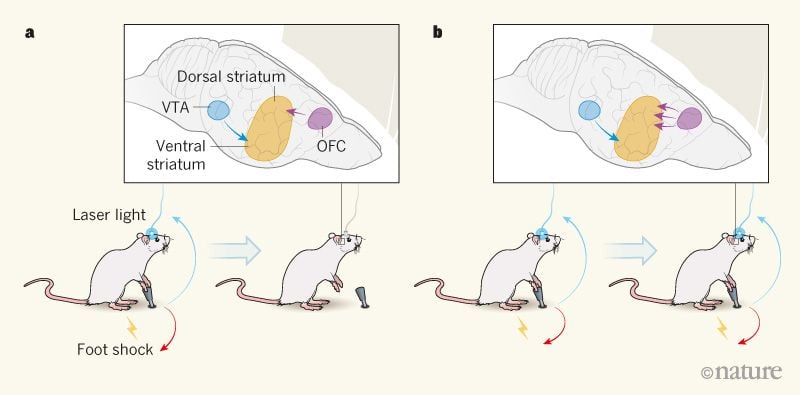
Figure 1 | Compulsive activation of dopamine neurons in the brain. In the study by Pascoli et al.1, mice pressed a lever to activate dopamine-releasing neurons through the delivery of laser light conducted by an optical fibre. These neurons, which project from the ventral tegmental area (VTA) to the ventral striatum in the brain, are associated with reward. a, Some mice, termed renouncers, reduced the lever-pressing behaviour when it was associated with a painful electric shock to their feet. The strength of the connections between neurons of the orbitofrontal cortex (OFC) projecting to the dorsal striatum was low in these mice. b, Other mice, termed perseverers, continued to press the lever despite the punishment — a hallmark of compulsive behaviour. The neural connections between the OFC and the dorsal striatum were stronger in these mice than in renouncers. When the authors weakened these connections in persevering mice, the animals’ compulsive behaviour decreased (not shown).
The authors next tried to determine what was different between the brains of perseverers and renouncers. They measured the activity of neurons connecting different brain areas in real time to determine which networks were active when mice pressed the lever. Communication between the orbitofrontal cortex (OFC), an area involved in decision-making, and the dorsal striatum, which is engaged in voluntary action, increased before lever-pressing in mice that were willing to obtain shocks along with dopamine self-stimulation. Optogenetic inhibition of this neural pathway turned persevering mice into renouncing mice. This finding shows that the increased activity of neurons projecting from the OFC to the dorsal striatum was necessary for this form of compulsive activation of dopamine neurons.
However, this behavioural switch was only temporary: when optogenetic inhibition was turned off, the compulsive behaviour resumed in persevering mice. The authors reasoned that long-lasting changes at the synapses — the junctions between neurons — that connect OFC and dorsal striatum neurons could arise as a result of the many days of self-stimulation of dopamine neurons. If these changes occurred only in persevering mice, this would explain their persistent compulsive behaviour.
If this hypothesis is true, the strength of synaptic connections between OFC and dorsal striatum neurons should be greater in perseverers than in renouncers, enabling better activation of dorsal striatum neurons by OFC neurons. Indeed, Pascoli et al. went on to show that the strength of the synapses between OFC neurons and dorsal striatum neurons had increased in persevering mice (Fig. 1). Renouncers, along with mice that had never been exposed to the experimental set-up and mice that received shocks but were not allowed to use the lever, all showed low synaptic strength between OFC and dorsal striatum neurons.
Remarkably, the authors found that compulsive behaviour could be suppressed or induced by respectively decreasing or increasing the strength of this neural connection. Weakening of the synaptic connections between the OFC and the dorsal striatum in persevering mice reduced their willingness to self-stimulate in the face of a possible foot shock. Conversely, renouncers could be turned into perseverers by increasing the strength of these synaptic connections. In contrast to the temporary reversal observed after optogenetic inhibition of OFC neurons projecting to the dorsal striatum, these changes in synaptic strength induced a behavioural switch that persisted for six days.
Pascoli et al. have discovered a neuroadaptation that allows mice to override a painful stimulus to continue activating their dopamine neurons. The chronic consumption of drugs of abuse in humans leads to repeated activation of the same dopamine-reinforcement circuit, so a similar neuroadaptation might cause them to continue taking drugs despite the negative consequences. To test this proposition, we should determine whether changes in the strength of the connections between OFC and dorsal striatum neurons mediate compulsive behaviour in mice pressing a lever to receive cocaine, amphetamines or opioids in the face of a possible foot shock.
Does the optogenetic stimulation of dopamine neurons accurately mimic the activation of dopamine neurons by drugs of abuse? There are obvious differences between quickly switching a laser on and off during optogenetic stimulation and the slower onset and longer duration of drug action. Nevertheless, the authors previously showed4 that cocaine intake and optogenetic activation induce almost identical adaptations in dopamine neurons and their immediate downstream targets, providing a strong rationale for the experimental approach used in the current study.
Why does the self-stimulation of dopamine neurons lead to compulsive behaviour in only a subset of individuals? Persevering and renouncing mice self-stimulated for approximately the same time and with a similar number of events before foot-shock punishments began, yet the brains of the two groups seem to have changed in divergent ways. The VTA dopamine neurons stimulated by the mice do not connect directly to the OFC or the dorsal striatum, so the link between these regions must involve multiple synaptic connections. A multisynaptic route through which the activation of VTA dopamine neurons might cause changes in the dorsal striatum has previously been described5, and has been proposed to underlie transitions from non-compulsive to compulsive drug-taking6,7. Pre-existing differences in this multisynaptic circuit might explain why compulsive behaviour, and the related changes in synaptic connections, occur in only some mice.
Synaptic changes can last for days, years or even a lifetime. Might the changes discovered by Pascoli et al. form the basis of an enduring behavioural change that is a hallmark of drug addiction? Resolving this question will require experimental evidence that drug self-administration despite negative consequences occurs through strengthening of the connections between the OFC and the dorsal striatum, and that it is indeed the activation of dopamine systems that sets in motion a chain of neural events that culminates in compulsive drug-taking.
Nature 564, 349-350 (2018)
doi: 10.1038/d41586-018-07716-z