Scientific Reports volume 9, Article number: 16918 (2019)
Abstract
Excessive sucrose consumption elicits addiction-like craving that may underpin the obesity epidemic. Opioids and dopamine mediate the rewarding effects of drugs of abuse, and of natural rewards from stimuli such as palatable food. We investigated the effects of sucrose using PET imaging with [11C]carfentanil (μ-opioid receptor agonist) and [11C]raclopride (dopamine D2/3 receptor antagonist) in seven female anesthetized Göttingen minipigs. We then gave minipigs access to sucrose solution for one hour on 12 consecutive days and performed imaging again 24 hours after the final sucrose access. In a smaller sample of five minipigs, we performed an additional [11C]carfentanil PET session after the first sucrose exposure. We calculated voxel-wise binding potentials (BPND) using the cerebellum as a region of non-displaceable binding, analyzed differences with statistical non-parametric mapping, and performed a regional analysis. After 12 days of sucrose access, BPND of both tracers had declined significantly in striatum, nucleus accumbens, thalamus, amygdala, cingulate cortex and prefrontal cortex, consistent with down-regulation of receptor densities. After a single exposure to sucrose, we found decreased binding of [11C]carfentanil in nucleus accumbens and cingulate cortex, consistent with opioid release. The lower availability of opioid and dopamine receptors may explain the addictive potential associated with intake of sucrose.
Introduction
Five percent of the world’s population are clinically obese1. As a hallmark of the metabolic syndrome, obesity is associated with type 2 diabetes, cardiovascular disease, respiratory problems, and risk of depression and possibly dementia2. The increased consumption of energy dense foods has exaggerated the physiologic distinction between homeostatic hunger that follows food deprivation, and hedonic hunger, or “craving”, which occurs in the absence of deprivation3,4. As the homeostatic regulation alone cannot account for the current rise in obesity, it is mandatory to test the effect on brain mechanisms of reward and pleasure of the addictive properties of highly palatable food.
Sucrose consumption is associated with obesity, and sucrose is increasingly considered an addictive substance5. Some findings are at variance with this claim due to difficulties in separating non-palatable food consumption from hedonic food responses, and in determining the addictive ingredient in processed food, as well as the different mechanisms by which food alters brain circuitry through natural pathways6. Nevertheless, in specific contexts, intake of sucrose does induce reward and craving, comparable in magnitude to those induced by addictive drugs, that lead to overconsumption and eventual obesity6,7.
Hunger is associated with “wanting” that is closely related to effects of dopaminergic neurotransmission in a number of reward circumstances8, but it remains unclear how the action of dopamine (DA) is modulated in response to compulsive eating. Consumption of palatable food is linked to “liking”, mediated primarily by the endogenous opioid system, especially the μ-opioid receptor (μOR)9,10, which can promote overconsumption when deregulated. In the present report, we test the claim that sucrose leads to opioid and dopamine release that lowers the availability of μOR and DA D2/3 receptors. The availability is an index of the number of unoccupied receptors available for tracer binding and in principle does not distinguish between ligand occupancy and receptor density11.
The onset of compulsive eating depends on multiple factors, and causal studies in humans raise ethical issues. The majority of studies therefore focus on feeding behavior in rats12. Although rats have a “sweet tooth”, their homeostatic mechanisms important to weight gain, metabolism, and type of fat accumulation, differ significantly from those of humans. The Göttingen minipig is a large omnivorous animal with a well-developed gyrencephalic brain, which can be imaged at sufficient resolution. Its well-defined subcortical and prefrontal cortical regions13 enable a more direct translation to human brain function. Here, we use positron emission tomography (PET) imaging to test in vivo μOR and DA D2/3 availability in a minipig model of subchronic sucrose exposure. In a smaller sample, we investigated the immediate effects on μOR occupancy after the first exposure to sucrose. Finally, we tested the relationship between the changes in receptor availability of the two tracers.
Results
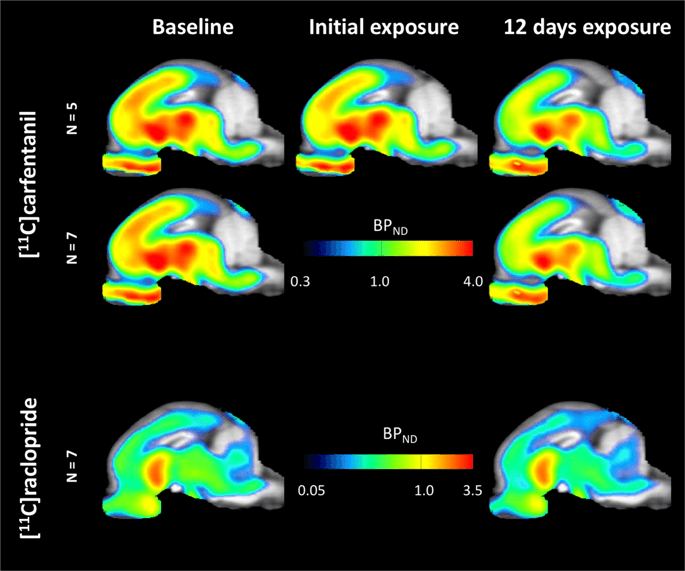
Average parametric maps of [11C]carfentanil and [11C]raclopride binding potential (BPND) are shown in Fig. 1. To analyze the changes occurring after the first sucrose exposure in five minipigs compared to baseline, and one day after the 12th sucrose access in seven minipigs compared to baseline, we used permutation theory and non-regionally restricted whole-brain analysis, the preferred method for samples of this size14.
Average voxel-wise non-displaceable binding potential (BPND) maps superimposed on MRI images in sagittal view. Data are presented for [11C]carfentanil BPND of the 5 minipigs imaged at baseline, after initial exposure to sucrose and after 12 days of sucrose exposure (top row). [11C]carfentanil BPND of all 7 minipigs imaged at baseline and after 12 days of sucrose access are presented in the middle row. [11C]raclopride BPND of all 7 minipigs imaged at baseline and after 12 days of sucrose access are shown in the bottom row. Note that the color scale is exponential to highlight the [11C]raclopride BPND in extrastriatal regions.
Initial sucrose exposure
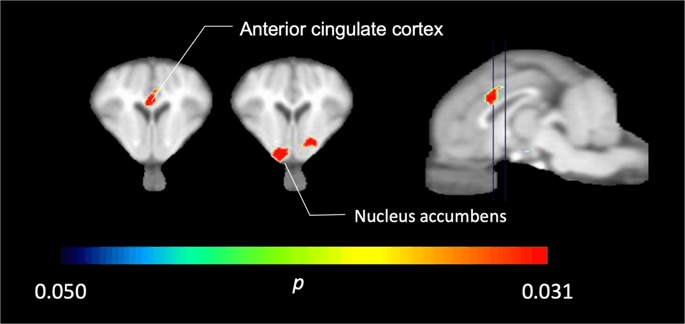
In the five minipigs imaged with [11C]carfentanil at baseline and immediately after the first sucrose exposure, we found significantly reduced tracer binding in the anterior cingulate cortex and the nucleus accumbens in response to sucrose, shown in color in Fig. 2, indicating p < 0.05. We detected as much as 14% decreased tracer binding in both areas compared to baseline.
Significant decreases in [11C]carfentanil BPND after the first sucrose water exposure compared to baseline (n = 5). Only voxels with significant (p < 0.05) decreases are shown as colored areas projected onto T1 weighted MRI cuts at the level of the anterior cingulate cortex (left) and nucleus accumbens (middle) from a stereotaxic minipig brain atlas. Note that the maximum significance level achievable with 5 animals is 2−5 ≈ 0.031 (see color bar). Data are presented on coronal sections of the pig brain at the levels indicated on the sagittal image (right).
12 days of sucrose access
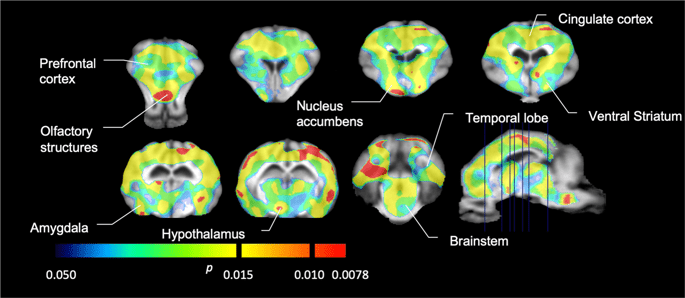
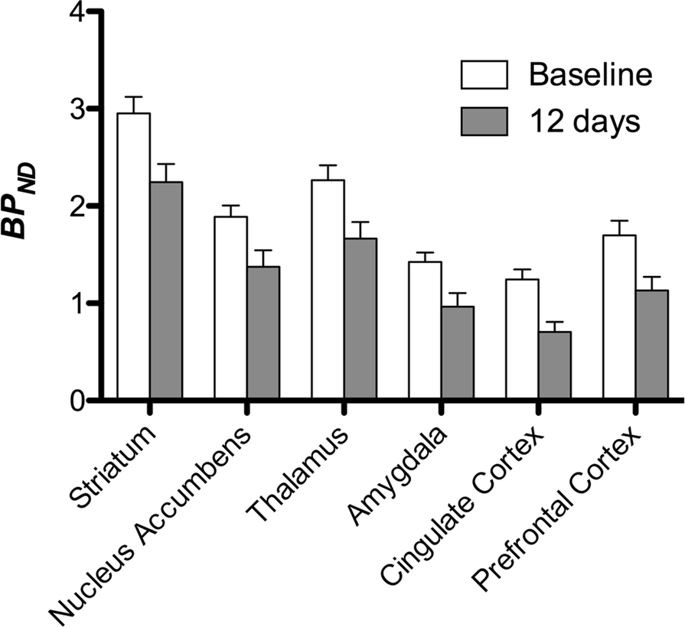
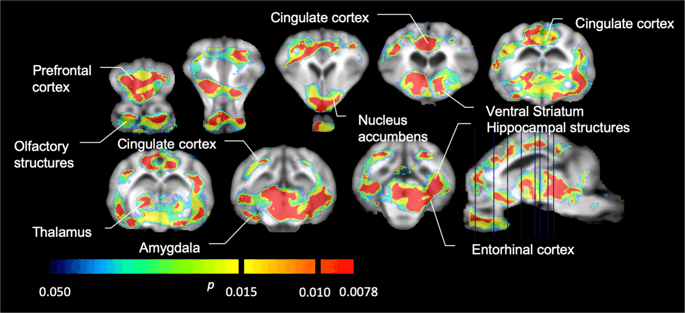
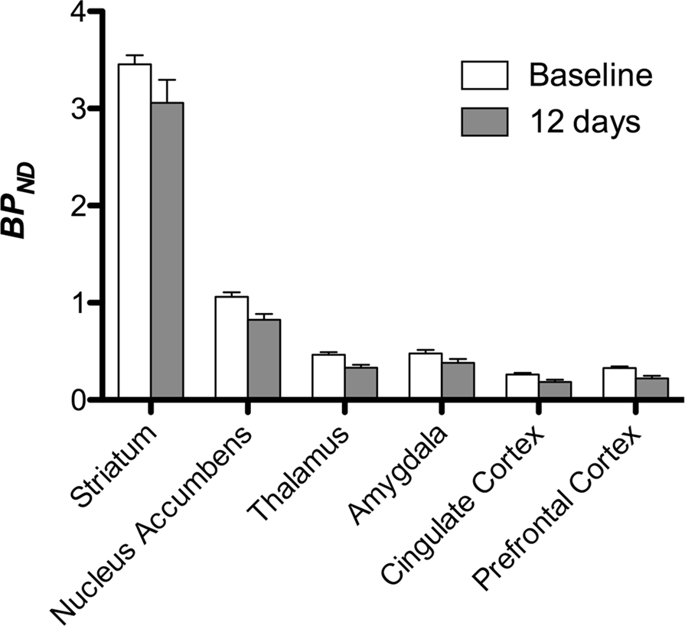
We then performed the analysis of seven minipigs imaged with [11C]carfentanil at baseline and after 12 days of sucrose access and found significantly reduced tracer binding in sucrose-exposed animals compared to baseline. The most highly significantly affected regions are shown in red in Fig. 3 (p < 0.01) and include parts of the olfactory structures, nucleus accumbens/ventral striatum and the temporal cortex/lobe, followed by areas shown in yellow (p < 0.015) which included parts of the prefrontal cortex, cingulate cortex, amygdala and brainstem. In order to obtain BPND values and assess percent change, we performed regional analysis and obtained mean values in each region at baseline and after sucrose consumption (Fig. 4).
Significant decreases in [11C]carfentanil binding potential (BPND) between baseline and after 12 days of sucrose water exposure (n = 7). The voxels with significant (p < 0.05) decreases are shown as colored areas projected onto T1 weighted MRI cuts from a stereotaxic minipig brain atlas. Data are presented on coronal brain sections at the levels indicated on the sagittal image (bottom right). Note that the maximum significance level achievable with 7 animals is 2−7 ≈ 0.0078 (see color bar).
We used [11C]raclopride as the tracer of DA D2/3 receptors in striatal and extrastriatal brain regions in minipigs at baseline and after 12 days of sucrose access (Fig. 1). We found decreased tracer binding in sucrose-exposed animals, compared to baseline with largest effects (p < 0.01) in areas of the prefrontal cortex, nucleus accumbens/ventral striatum, cingulate cortex, amygdala, thalamus, mesencephalon, hippocampal regions, and olfactory areas (Fig. 5). Data from regional analysis are presented in Fig. 6.
Significant decreases in [11C]raclopride binding potential (BPND) between baseline and after 12 days of sucrose water exposure (n = 7). The voxels with significant (p < 0.05) decreases are shown as colored areas projected onto T1 weighted MRI cuts from a stereotaxic minipig brain atlas. Data are presented on coronal sections of the pig brain at the levels indicated on the sagittal image (bottom right). Note that the maximum significance level achievable with 7 animals is 2−7 ≈ 0.0078 (see color bar).
Correlations between [11C]raclopride and [11C]carfentanil data
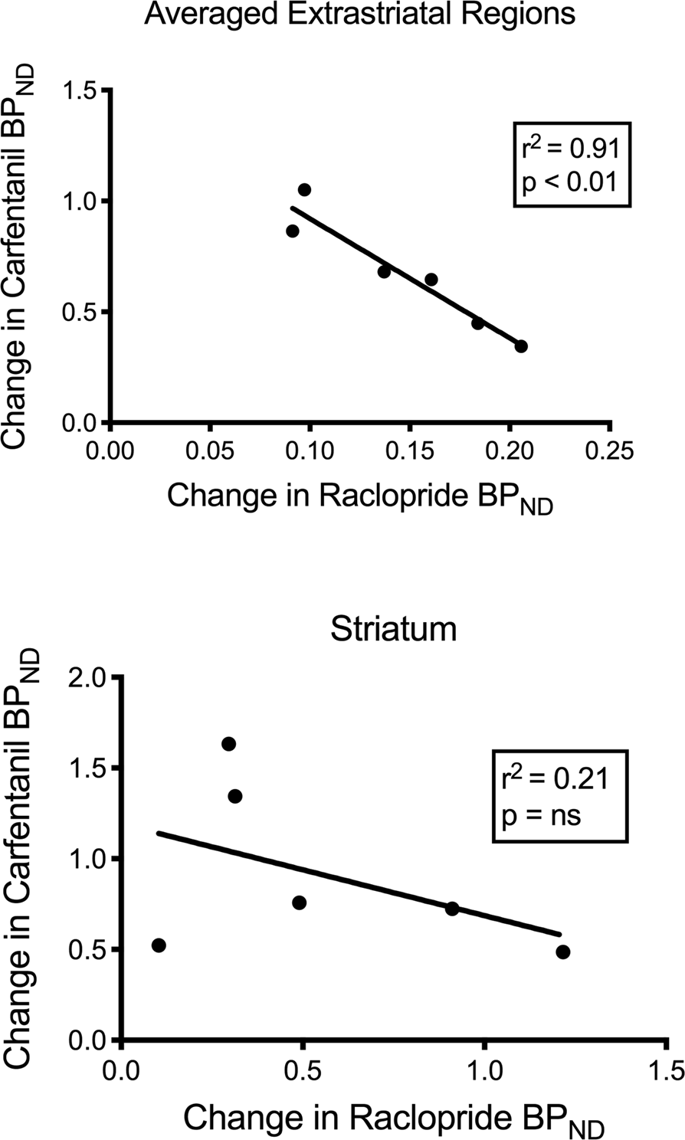
We tested the potential correlation between [11C]raclopride and [11C]carfentanil values of BPND in striatal and non-striatal regions in minipigs at baseline and after 12 days of sucrose intake, with no associations observed. We then tested whether declines of tracer binding were correlated, and we compared the changes of BPND for [11C]raclopride with the changes of BPND for [11C]carfentanil only in the minipigs that had lower BPND of both tracers after sucrose intake (n = 6). We found significant negative correlations in averaged extrastriatal (r2 = 0.91, p < 0.01), but not in striatal, regions (Fig. 7).
Correlations between pre- minus post- declines of [11C]raclopride and [11C]carfentanil binding potentials (BPND) in minipigs with decreased tracer binding after sucrose intake (n = 6). Data from the averaged extrastriatal regions (top) and striatum (bottom) are presented. The coefficient of determination (r2) and the p values are shown for each graph.
Discussion
We determined the effects of repeated intermittent access to sucrose on opioid and DA neurotransmission in mammalian brain. Longitudinal in vivo PET imaging of the μOR and DA D2/3 receptors revealed reduced receptor availability throughout the reward circuit, including the nucleus accumbens, prefrontal cortex, and the anterior cingulate cortex. The results clearly demonstrate that sucrose affects reward mechanisms in a manner similar to that of drugs of abuse.
The intake of sucrose as a palatable substance is known to release DA and induce dependency in rodents15, with sucrose shown to be even more pleasurable than cocaine in rodents in certain contexts. Thus, rodents work more intensely to obtain sucrose than cocaine, even in the absence of food deprivation5. However, the effects of sucrose are regulated both by the homeostatic system and by hedonic reward circuits16,17 that may mediate the distinction between nutritional and hedonic aspects of sucrose action18. We opted for a one-hour per day schedule in order to promote “binging”, as previous studies in rats had revealed a higher intake during the first hour of daily access in an intermittent schedule15,19. Behavioral studies of food intake often target food-restricted animals, but the design may not necessarily reflect the same neural mechanisms active in obesity. Pigs in the present study were not food restricted and were fed the usual amounts of their normal diet in addition to access to sucrose.
Opioid receptors (OR) are widely expressed in the brain, specifically in structures known to modulate eating and reward processes20. ORs have been shown to be important in the rewarding and relapsing effects of cocaine21,22,23,24. Alterations in binding have also been linked to the homeostatic responses to eating and the pleasure associated with palatable food25. In particular, “liking” of food is linked to the endogenous opioid system, especially the μOR9,10 in the shell of the nucleus accumbens and the ventral pallidum26. Infusions of a μOR agonist into distinct portions of the nucleus accumbens and ventral pallidum strongly enhance “liking” behaviors, including tongue protrusions and paw licking, following increased palatable intake of food27,28,29. Further evidence for opioid signaling in the processing of hedonic regulation comes from μOR antagonists that attenuate consumption of palatable chow in both ad libitum-fed and food restricted animals, but with a more limited effect on the intake of non-palatable standard pellets30,31. In humans, μOR antagonists decrease short-term food intake and reduce pleasantness of palatable foods32,33,34. Opioid signaling in the basolateral amygdala also contributes to food “wanting” through modulation of reward seeking and the incentive value of food35.
With [11C]carfentanil, we obtained images of tracer binding that is sensitive both to μOR levels and to the brain’s release of endogenous opioids36,37. We detected immediate loss of μOR availability in areas of the nucleus accumbens and anterior cingulate cortex, specific brain regions of the reward pathway, after initial consumption of sucrose by five minipigs, consistent with endogenous opioid release. Previous studies have shown that palatable food can lead to feelings of pleasure38 by stimulating opioid release. After 12 days of sucrose access, we observed decreased [11C]carfentanil binding, which has several possible explanations39 including endogenous opioid release and binding to μOR, μOR internalization as a result of increased opioid binding, and increased DA D2/3 receptor activation leading to heterologous desensitization of μOR40.
In support of the present findings, [11C]carfentanil studies of patients with bulimia41, obesity42,43,44, and binge-eating disorder45, show decreased receptor availability. However, these are chronic conditions whereas the minipigs only received sucrose for 12 days. In a study of acute feeding behavior in healthy men, feeding led to robust and widespread endogenous cerebral opioid release, both in the presence and absence of hedonia, suggesting that opioid release reflects metabolic and homeostatic, as well as hedonic, responses25. This study, together with another that imaged patients after a chocolate-flavored liquid meal44, is directly relevant to the acute study of five minipigs after the first sucrose exposure, but is different from the subchronic sucrose-exposure study over 12 days where the reduced receptor availability more likely reflects repeated overstimulation and concomitant downregulation of μOR.
The prefrontal cortex is important in decision-making and ascribing value to items and therefore the μOR in the prefrontal cortex may be accountable for the altered evaluation of food saliency, which can raise the addictive potential of food. We have found decreased binding in the prefrontal cortex, consistent with previous studies showing that high fat diet reduces levels of μOR mRNA in the prefrontal cortex46 and that infusion of a μOR agonist in the prefrontal cortex increases intake of sweet food47. Again, however, the issue arises whether the high fat diet is a more chronic condition that more likely mediates receptor down-regulation, compared to the shorter-term sucrose-feeding design, suggesting sustained release of endogenous opioids that displaces tracer carfentanil bound to μOR, even after 12 days of sucrose.
DA has been implicated in rewards both from drugs and behavior. Chronic cocaine use has been found to inhibit DA signaling48. DA D1 and D2/3 receptor levels are altered by nicotine in pig brain49, and in non-human primates with a history of cocaine abuse50, consistent with the downregulation of D2/3 receptors in the brains of human cocaine addicts51,52. As for drugs of abuse, sucrose has been shown to upregulate DA D1 receptors19 and increase DA release53, reinforcing the role of DA in “wanting” in relation to palatable food. Previous PET studies have demonstrated a decrease in striatal DA D2/3 receptor availability in morbid obesity vs average weight54,55, similar in magnitude to the reduction in drug-addicted patients56, and in animal with models of obesity57. In rodent studies, D2/3 receptor knockdown in the striatum promotes the development of compulsive food seeking in rats with access to palatable food57.
Our observations of decreased D2/3 receptor availability of the pig may indicate increased DA levels in response to the incentive salience associated with the sucrose intake since DA is released as part of the wanting of drugs of abuse and other pleasurable activities52,58,59,60. As the pigs were anesthetized during the imaging, and had not received sucrose in 24 hours, the decreased D2/3 BPND more likely reflects a reduction in the number of receptors in response to prolonged increase of DA release at each of the 12 days of sucrose access. The reduction can raise brain reward thresholds, associated with down-regulation of striatal DA D2 receptors. This may explain the increased susceptibility to drugs of abuse seen in previous studies of rats overeating sucrose that led to cross-sensitization to cocaine, hyperactivity after low dose amphetamine, increased alcohol intake when abstaining from sucrose, and tolerance to the analgesic effects of opiates6.
A previous study of obesity in the Göttingen minipig identified decreased cerebral blood flow in the nucleus accumbens, ventral tegmental area (VTA) and prefrontal cortex, with single photon emission computed tomography (SPECT) of brain61. Consistent with these findings, we observed reduced DA D2/3 binding in the ventroforebrain region containing the nucleus accumbens and in the prefrontal cortex. Extracellular levels of DA are increased 3-fold in the nucleus accumbens after sucrose intake in freely-moving rats undergoing microdialysis62. In sucrose dependent animals, repeated sucrose intake can lead to release of DA from the shell of nucleus accumbens63. Animals fed a restricted diet with limited access to sucrose had lower DA D2 receptor binding in the nucleus accumbens shell and the dorsal striatum64. Restricted high fat and sucrose diets can lead to sustained downregulation of D1 and D2 receptor mRNA in the nucleus accumbens65. A microdialysis study of the effects of palatable food revealed increased DA release in the nucleus accumbens and prefrontal cortex when the food was still considered novel; once the rats were accustomed to the new food, the increased release was blunted in the nucleus accumbens, but not in the prefrontal cortex66. The differential susceptibility to habituation and conditioning of the activity in two regions may explain the larger increase observed in prefrontal cortex than in nucleus accumbens of minipigs exposed to the same palatable substance that lost novelty after twelve days. However, as we did not image minipigs with [11C]raclopride after the first sucrose administration, this explanation is speculative.
The prefrontal cortex modulates executive function, decision-making, and self-control67. Dysfunctional DA neurotransmission in the prefrontal cortex impairs modulation of reward processing, suggesting impaired executive function and decision-making skills in obese individuals68,69. Moreover, a human PET study correlated decreased frontal cortex metabolism with decreased striatal D2 binding in obesity70. Here, we find reduced D2/3 receptor availability in the prefrontal cortex including the orbitofrontal cortex of pigs exposed to the sucrose regimen.
Dopaminergic neurons of the VTA send projections to the hippocampus and amygdala, where they support habit-like behaviors71 and mediate the encoding and retrieval of conditioning to drug72,73 and food cues74,75. Human brain imaging has shown hippocampal activation in response to food craving and tasting76. Consistent with our findings of a reduced hippocampal and amygdalar D2/3 receptor availability in response to sucrose, human brain mapping with [18F]fallypride showed cocaine cue-induced DA release in amygdala and hippocampus77. In rodent brains, cocaine cue exposure triggered DA release in the amygdala78, and alterations of amygdala DA levels influenced cue-induced cocaine-seeking behavior79.
In a study of obese individuals, the association between D2/3 and μOR availabilities, known to exist in striatal regions of lean individuals, was disrupted in the ventral striatum80. We compared the values of BPND of the two tracers to test if the data reproduced this effect. Unlike lean humans, the present brains of pigs had no correlation between the values of BPND of the two tracers, at baseline or after the exposure to sucrose. We then tested whether the animals with the largest declines of tracer raclopride binding would also have the largest decreases of tracer carfentanil binding, but instead we found a negative correlation in the averaged extrastriatal regions, suggesting that animals with the greatest change of the binding potential of tracer raclopride had the lowest change of the binding potential of tracer carfentanil. The inverse relation between the changes suggests that the effects of sucrose intake on the availabilities of the respective receptors are regulated in opposite directions. It is known that excessive consumption of palatable food, or drugs, can be driven by wanting or liking, or both60,81. It is possible that the magnitude of wanting driven by dopamine negates the magnitude of liking driven by opioids, or vice versa. Recent evidence points to roles of GABAA receptors in the VTA and cholinergic terminals in striatum and possibly cortex that act as switches between dopamine-dependent and dopamine-independent mechanisms of opioid action82,83 that may explain the reciprocity of dopamine and opioid effects in porcine extrastriatal regions determined here.
A shortcoming of PET, also in comparatively large animals, is the limited spatial resolution of the tomography that affects the results from small brain regions involved in food-associated behaviors. However, despite these concerns, [11C]raclopride binding previously was recorded both in striatal and extrastriatal regions84,85,86,87. The use of [11C]raclopride to label the same type of receptors raises no concern about potential affinity differences that may affect the use of separate tracers for the same receptors in different regions. Recent studies included records of extrastriatal binding of [11C]raclopride. Alakurtti et al. found good reproducibility of measures of striatal raclopride binding in the striatum, with only good to moderate reproducibility in the cortex85. In a later study, Svensson et al. discussed several issues affecting the use of [11C]raclopride as a marker of extrastriatal D2/3 receptors in a study of healthy humans, including poor reproducibility in cortex and limited decline of extrastriatal binding in frontal cortex in response to a D2/3 blocking agent88. The test-retest comparisons revealed variabilities of 4–7% in striatum and 13–59% in cortical regions, but the time between examinations averaged 20 days, unlike the more informative 1–2 days of most studies. A number of factors in the lives of those subjects may have had time to influence the findings. Indeed, we show here that merely adding sucrose consumption to a morning routine for 12 days may have influenced binding measures obtained two weeks later. Other factors as common as playing video games, shopping, entering new romantic relationships and sexual activity, using drugs or changing diet and exercise may influence extrastriatal dopamine levels with potential for great variation of datasets. The current study in minipigs introduced a well-controlled set-up with the only variable being the absence or presence of sucrose in the diet. In this context, the data from seven animals had sufficiently low variability in relevant extrastriatal regions to identify a statistically significant reduction of binding in response to sucrose.
A limitation of the current study is the use of anaesthetics required to ensure immobility during in vivo imaging of animals. The effects of specific anaesthetics, and their interactions with drugs or other interventions, can confound the binding of radioligands89,90. Ketamine is an anti-glutamatergic drug with rapid antidepressant effects in sub-anaesthetic doses91,92,93, that do not reduce striatal [11C]raclopride binding in humans94. However, S-ketamine was found to reduce binding availability of dopamine D2/3 receptors in striatum of conscious non-human primates95. Isoflurane is a common anaesthetic in animal PET. In previous studies, we found striatal accumulation of [11C]SCH23390, a radioligand of the dopamine D1 receptors to be significantly higher in minipigs anesthetized with isoflurane rather than propofol, suggesting susceptibility of the dopaminergic neurotransmission to effects of anaesthesia96. In the current study, all minipigs were imaged at both timepoints under ketamine pre-medication and isoflurane anaesthesia, making the present comparisons valid.
Conclusion
Excessive consumption of palatable food may both cause, and become the result of, addiction with direct consequences to health by obesity. We tested the claim that opioids and dopamine mediate rewards, important to survival as well as to abuse of drugs. Minipigs with intermittent access to a sucrose solution on 12 consecutive days demonstrated decreased dopamine D2/3 and μ−opioid receptor availability in striatal and extrastriatal brain regions, implying that foods high in sucrose influence brain reward circuitry in ways similar to those observed when addictive drugs are consumed. Initial single exposure to sucrose was consistent with opioid release in brain regions active in reward. The changes of opioid and dopamine availability explain the addictive potential of sucrose consumed in excess.
Materials and Methods
Animal ethics
This study was approved and regulated by the Danish Animal Experiments Inspectorate and all experiments were carried out in accordance with the 2010/63/EU directive of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes and the ARRIVE guidelines. We used seven fourteen-month old female Göttingen minipigs (Ellegaard, Dalmose, Denmark). Minipigs were fed a pellet diet (6 dL, 2 times daily, Special Diets Services, Aarhus, Denmark) with tap-water available ad libitum. The environmental temperature was 20–22 °C, relative humidity 50–55%, and air was changed eight times every hour.
Intermittent sucrose consumption
We imaged seven minipigs with [11C]raclopride and [11C]carfentanil at baseline, and again one day after 12 consecutive days of sucrose water exposure. Sucrose exposure consisted of one hour of sucrose (sucrose, Dansukker, Copenhagen, Denmark) water access (500 grams of sucrose in 2 liters of water), daily during a 12-day period. The amount of sucrose intake was recorded and all minipigs consumed 2 liters on each day. We also imaged five of the same minipigs with [11C]carfentanil, 30 minutes after the first sucrose access, in order to study acute opioid release.
The minipigs gained an average of 13.6% body weight from 25.4 kg (±0.73 SEM) at baseline to 28.9 kg (±0.69 SEM) after the 12-day sucrose exposure, which was significantly higher (one-tailed t-test, p < 0.001) than the increases observed in a sample of control minipigs obtained in previous studies, where weights increased on average by only 4.9%, during the same developmental period.
Brain PET Imaging
We fasted pigs overnight with free access to water prior to imaging. We pre-medicated and anesthetized minipigs as described previously97 and placed them supine in a PET/CT device (Siemens Biograph 64 Truepoint PET). We performed a low-dose CT scan prior to each PET acquisition for anatomical definition and attenuation correction of PET emission data. We intravenously administered [11C]raclopride at baseline (360 ± 18 MBq, specific activity 77 ± 76 GBq/μmol, injected mass 0.12 ± 0.08 μg/kg) and after 12 days of sucrose (374 ± 54 MBq, specific activity 127 ± 85 GBq/μmol, injected mass 0.06 ± 0.05 μg/kg), and [11C]carfentanil at baseline (377 ± 43 MBq, specific activity 311 ± 195 GBq/μmol, injected mass 0.03 ± 0.02 μg/kg) and after 12 days of sucrose (337 ± 71 MBq, specific activity 177 ± 157 GBq/μmol, injected mass 0.06 ± 0.08 μg/kg) via ear vein, in 10 mL saline, during the first minute of a 90-minute scan. We reconstructed PET data using TrueX 3D OSEM (3 iterations, 21 subsets), a 256 × 256 × 109 matrix, and a 2-mm Gauss filter, using a time-frame structure of 5 × 60, 3 × 300, 4 × 600, 2 × 900 seconds (total 14 frames, 90 minutes). At baseline and after 12 days of sucrose, minipigs were imaged with both tracers injected at least 100 minutes apart, due to the half-life of [11C] PET tracers. Upon completion of the final PET session, we euthanized minipigs under deep anesthesia by an intravenous overdose of pentobarbital (100 mg/kg).
Quantitative analyses and statistics
We performed preprocessing steps using PMOD 3.7 (PMOD Technologies Ltd, Zurich, Switzerland). To define the stereotactic transformation parameters from time-averaged PET images, we used ligand-specific templates. We applied the generated transformation matrices and warping fields onto the corresponding dynamic PET time series. We generated parametric images of [11C]raclopride binding potential (BPND) by means of the multilinear reference tissue method of Ichise and co-workers98. We created a custom-made mask of the cerebellum that excluded the vermis to obtain the cerebellar tissue radioactivity over time in a region of negligible DA D2/3 receptor density. We generated parametric images of [11C]carfentanil using an implementation of the Logan reference tissue model99,100 with t* = 30 min. Studies of [11C]carfentanil binding in human brain have used the occipital cortex as a reference region36; however, in the pig, according to the time activity curves, non-displaceable binding was lower in the cerebellum than in the occipital cortex, consistent with findings from a rat autoradiography study101. We therefore selected the cerebellum as the reference region in the current study.
Statistical analysis
We subjected maps to a voxel-wise analysis with Statistical Non-Parametric Mapping (SnPM v13.01, http://warwick.ac.uk/snpm) SPM toolbox that utilizes non-parametric permutation theory to provide a framework for statistical inference, an approach shown to work well for small samples due to strict control of false positives14 and applied as previously described102. An expert in pig neuroanatomy (DO) compared the resulting images thresholded to 5% significance level to a high-resolution Göttingen minipig atlas103,104 to define and label regions of decreased DA D2/3 and μOR BPND from baseline to the post-sucrose condition. We then performed a region-of-interest (ROI) analysis in order to extract BPND values of specific regions found to be of interest based on the SnPM analysis, including the striatum, nucleus accumbens, thalamus, amygdala, cingulate cortex and prefrontal cortex. No additional statistics were performed on the ROI analysis, since these regions were already found to be significant using SnPM.
References
- 1.
Smyth, S. & Heron, A. Diabetes and obesity: the twin epidemics. Nat Med 12, 75–80, https://doi.org/10.1038/nm0106-75 (2006).
- 2.
Flegal, K. M., Carroll, M. D., Ogden, C. L. & Curtin, L. R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241, https://doi.org/10.1001/jama.2009.2014 (2010).
- 3.
Davis, C. A. et al. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 17, 1220–1225, https://doi.org/10.1038/oby.2009.52 (2009).
- 4.
Drewnowski, A. Obesity and the food environment: dietary energy density and diet costs. American journal of preventive medicine 27, 154–162, https://doi.org/10.1016/j.amepre.2004.06.011 (2004).
- 5.
Lenoir, M., Serre, F., Cantin, L. & Ahmed, S. H. Intense sweetness surpasses cocaine reward. PloS one 2, e698, https://doi.org/10.1371/journal.pone.0000698 (2007).
- 6.
Ahmed, S., Avena, N. M., Berridge, K. C., Gearhardt, A. & Guillem, K. In Neuroscience in the 21st Century (ed. Phaff, D. W.) (Springer, 2012).
- 7.
Avena, N. M., Gold, J. A., Kroll, C. & Gold, M. S. Further developments in the neurobiology of food and addiction: update on the state of the science. Nutrition 28, 341–343, https://doi.org/10.1016/j.nut.2011.11.002 (2012).
- 8.
Leyton, M. In Pleasures of the Brain (eds Kringelbach, M. L. & Berridge, K. C.) (Oxford University Press, 2010).
- 9.
Nathan, P. J. & Bullmore, E. T. From taste hedonics to motivational drive: central mu-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol 12, 995–1008, https://doi.org/10.1017/S146114570900039X (2009).
- 10.
Berridge, K. C. Food reward: brain substrates of wanting and liking. Neuroscience and biobehavioral reviews 20, 1–25 (1996).
- 11.
Gjedde, A., Wong, D. F., Rosa-Neto, P. & Cumming, P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int Rev Neurobiol 63, 1–20, https://doi.org/10.1016/S0074-7742(05)63001-2 (2005).
- 12.
Avena, N. M., Bocarsly, M. E. & Hoebel, B. G. Animal models of sugar and fat bingeing: relationship to food addiction and increased body weight. Methods Mol Biol 829, 351–365, https://doi.org/10.1007/978-1-61779-458-2_23 (2012).
- 13.
Jelsing, J. et al. The prefrontal cortex in the Gottingen minipig brain defined by neural projection criteria and cytoarchitecture. Brain Res Bull 70, 322–336, https://doi.org/10.1016/j.brainresbull.2006.06.009 (2006).
- 14.
Nichols, T. E. & Holmes, A. P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15, 1–25 (2002).
- 15.
Avena, N. M., Rada, P. & Hoebel, B. G. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neuroscience and biobehavioral reviews 32, 20–39, https://doi.org/10.1016/j.neubiorev.2007.04.019 (2008).
- 16.
Alonso-Alonso, M. et al. Food reward system: current perspectives and future research needs. Nutr Rev 73, 296–307, https://doi.org/10.1093/nutrit/nuv002 (2015).
- 17.
Figlewicz, D. P., Bennett-Jay, J. L., Kittleson, S., Sipols, A. J. & Zavosh, A. Sucrose self-administration and CNS activation in the rat. Am J Physiol Regul Integr Comp Physiol 300, R876–884, https://doi.org/10.1152/ajpregu.00655.2010 (2011).
- 18.
Tellez, L. A. et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nature neuroscience 19, 465–470, https://doi.org/10.1038/nn.4224 (2016).
- 19.
Colantuoni, C. et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport 12, 3549–3552 (2001).
- 20.
Pert, C. B., Kuhar, M. J. & Snyder, S. H. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci USA 73, 3729–3733 (1976).
- 21.
Soderman, A. R. & Unterwald, E. M. Cocaine reward and hyperactivity in the rat: sites of mu opioid receptor modulation. Neuroscience 154, 1506–1516, https://doi.org/10.1016/j.neuroscience.2008.04.063 (2008).
- 22.
Ward, S. J., Martin, T. J. & Roberts, D. C. Beta-funaltrexamine affects cocaine self-administration in rats responding on a progressive ratio schedule of reinforcement. Pharmacology, biochemistry, and behavior 75, 301–307 (2003).
- 23.
Schroeder, J. A. et al. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology 195, 265–272, https://doi.org/10.1007/s00213-007-0883-z (2007).
- 24.
Tang, X. C., McFarland, K., Cagle, S. & Kalivas, P. W. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. The Journal of neuroscience: the official journal of the Society for Neuroscience 25, 4512–4520, https://doi.org/10.1523/JNEUROSCI.0685-05.2005 (2005).
- 25.
Tuulari, J. J. et al. Feeding Releases Endogenous Opioids in Humans. J Neurosci 37, 8284–8291, https://doi.org/10.1523/JNEUROSCI.0976-17.2017 (2017).
- 26.
Smith, K. S. & Berridge, K. C. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27, 1594–1605, https://doi.org/10.1523/JNEUROSCI.4205-06.2007 (2007).
- 27.
Pecina, S. & Berridge, K. C. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863, 71–86 (2000).
- 28.
Zhang, M. & Kelley, A. E. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology 159, 415–423, https://doi.org/10.1007/s00213-001-0932-y (2002).
- 29.
Zhang, M., Gosnell, B. A. & Kelley, A. E. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. The Journal of pharmacology and experimental therapeutics 285, 908–914 (1998).
- 30.
Levine, A. S., Weldon, D. T., Grace, M., Cleary, J. P. & Billington, C. J. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol 268, R248–252 (1995).
- 31.
Glass, M. J., Billington, C. J. & Levine, A. S. Opioids and food intake: distributed functional neural pathways? Neuropeptides 33, 360–368, https://doi.org/10.1054/npep.1999.0050 (1999).
- 32.
Fantino, M., Hosotte, J. & Apfelbaum, M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol 251, R91–96, https://doi.org/10.1152/ajpregu.1986.251.1.R91 (1986).
- 33.
Arbisi, P. A., Billington, C. J. & Levine, A. S. The effect of naltrexone on taste detection and recognition threshold. Appetite 32, 241–249, https://doi.org/10.1006/appe.1998.0217 (1999).
- 34.
Drewnowski, A., Krahn, D. D., Demitrack, M. A., Nairn, K. & Gosnell, B. A. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr 61, 1206–1212 (1995).
- 35.
Wassum, K. M., Ostlund, S. B., Maidment, N. T. & Balleine, B. W. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc Natl Acad Sci USA 106, 12512–12517, https://doi.org/10.1073/pnas.0905874106 (2009).
- 36.
Colasanti, A. et al. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry 72, 371–377, https://doi.org/10.1016/j.biopsych.2012.01.027 (2012).
- 37.
Mick, I. et al. Amphetamine induced endogenous opioid release in the human brain detected with [11C]carfentanil PET: replication in an independent cohort. Int J Neuropsychopharmacol, 1–6, https://doi.org/10.1017/S1461145714000704 (2014).
- 38.
Yeomans, M. R. & Gray, R. W. Opioid peptides and the control of human ingestive behaviour. Neuroscience and biobehavioral reviews 26, 713–728 (2002).
- 39.
Sprenger, T., Berthele, A., Platzer, S., Boecker, H. & Tolle, T. R. What to learn from in vivo opioidergic brain imaging? Eur J Pain 9, 117–121, https://doi.org/10.1016/j.ejpain.2004.07.010 (2005).
- 40.
Unterwald, E. M. & Cuntapay, M. Dopamine-opioid interactions in the rat striatum: a modulatory role for dopamine D1 receptors in delta opioid receptor-mediated signal transduction. Neuropharmacology 39, 372–381 (2000).
- 41.
Bencherif, B. et al. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 46, 1349–1351 (2005).
- 42.
Karlsson, H. K. et al. Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci 35, 3959–3965, https://doi.org/10.1523/JNEUROSCI.4744-14.2015 (2015).
- 43.
Karlsson, H. K. et al. Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Mol Psychiatry 21, 1057–1062, https://doi.org/10.1038/mp.2015.153 (2016).
- 44.
Burghardt, P. R., Rothberg, A. E., Dykhuis, K. E., Burant, C. F. & Zubieta, J. K. Endogenous Opioid Mechanisms Are Implicated in Obesity and Weight Loss in Humans. J Clin Endocrinol Metab 100, 3193–3201, https://doi.org/10.1210/jc.2015-1783 (2015).
- 45.
Majuri, J. et al. Dopamine and Opioid Neurotransmission in Behavioral Addictions: A Comparative PET Study in Pathological Gambling and Binge Eating. Neuropsychopharmacology 42, 1169–1177, https://doi.org/10.1038/npp.2016.265 (2017).
- 46.
Vucetic, Z., Kimmel, J. & Reyes, T. M. Chronic high-fat diet drives postnatal epigenetic regulation of mu-opioid receptor in the brain. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 36, 1199–1206, https://doi.org/10.1038/npp.2011.4 (2011).
- 47.
Mena, J. D., Sadeghian, K. & Baldo, B. A. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience 31, 3249–3260, https://doi.org/10.1523/JNEUROSCI.2050-10.2011 (2011).
- 48.
Park, K., Volkow, N. D., Pan, Y. & Du, C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience 33, 15827–15836, https://doi.org/10.1523/JNEUROSCI.1935-13.2013 (2013).
- 49.
Cumming, P. et al. Effects of acute nicotine on hemodynamics and binding of [11C]raclopride to dopamine D2,3 receptors in pig brain. NeuroImage 19, 1127–1136 (2003).
- 50.
Moore, R. J., Vinsant, S. L., Nader, M. A., Porrino, L. J. & Friedman, D. P. Effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse 30, 88–96, doi:10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L (1998).
- 51.
Volkow, N. D. et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of neuroscience: the official journal of the Society for Neuroscience 26, 6583–6588, https://doi.org/10.1523/JNEUROSCI.1544-06.2006 (2006).
- 52.
Wong, D. F. et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 31, 2716–2727, https://doi.org/10.1038/sj.npp.1301194 (2006).
- 53.
Hajnal, A., Smith, G. P. & Norgren, R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286, R31–37, https://doi.org/10.1152/ajpregu.00282.2003 (2004).
- 54.
Volkow, N. D., Fowler, J. S., Wang, G. J., Baler, R. & Telang, F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56(Suppl 1), 3–8, https://doi.org/10.1016/j.neuropharm.2008.05.022 (2009).
- 55.
Wang, G. J. et al. Brain dopamine and obesity. Lancet 357, 354–357 (2001).
- 56.
Wang, G. J., Volkow, N. D., Thanos, P. K. & Fowler, J. S. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. Journal of addictive diseases 23, 39–53, https://doi.org/10.1300/J069v23n03_04 (2004).
- 57.
Johnson, P. M. & Kenny, P. J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature neuroscience 13, 635–641, https://doi.org/10.1038/nn.2519 (2010).
- 58.
Berridge, K. C. & Kringelbach, M. L. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology 199, 457–480, https://doi.org/10.1007/s00213-008-1099-6 (2008).
- 59.
Berridge, K. C. & Kringelbach, M. L. Pleasure systems in the brain. Neuron 86, 646–664, https://doi.org/10.1016/j.neuron.2015.02.018 (2015).
- 60.
Schultz, W. Predictive reward signal of dopamine neurons. J Neurophysiol 80, 1–27, https://doi.org/10.1152/jn.1998.80.1.1 (1998).
- 61.
Val-Laillet, D., Layec, S., Guerin, S., Meurice, P. & Malbert, C. H. Changes in brain activity after a diet-induced obesity. Obesity 19, 749–756, https://doi.org/10.1038/oby.2010.292 (2011).
- 62.
Hajnal, A. & Norgren, R. Accumbens dopamine mechanisms in sucrose intake. Brain Res 904, 76–84 (2001).
- 63.
Rada, P., Avena, N. M. & Hoebel, B. G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 134, 737–744, https://doi.org/10.1016/j.neuroscience.2005.04.043 (2005).
- 64.
Bello, N. T., Lucas, L. R. & Hajnal, A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport 13, 1575–1578 (2002).
- 65.
Alsio, J. et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 171, 779–787, https://doi.org/10.1016/j.neuroscience.2010.09.046 (2010).
- 66.
Bassareo, V. & Di Chiara, G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci 17, 851–861 (1997).
- 67.
Volkow, N. D., Wang, G. J., Tomasi, D. & Baler, R. D. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol 23, 639–648, https://doi.org/10.1016/j.conb.2013.01.002 (2013).
- 68.
Brogan, A., Hevey, D. & Pignatti, R. Anorexia, bulimia, and obesity: shared decision making deficits on the Iowa Gambling Task (IGT). J Int Neuropsychol Soc 16, 711–715, https://doi.org/10.1017/S1355617710000354 (2010).
- 69.
Davis, C., Levitan, R. D., Muglia, P., Bewell, C. & Kennedy, J. L. Decision-making deficits and overeating: a risk model for obesity. Obes Res 12, 929–935, https://doi.org/10.1038/oby.2004.113 (2004).
- 70.
Volkow, N. D. et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage 42, 1537–1543, https://doi.org/10.1016/j.neuroimage.2008.06.002 (2008).
- 71.
Lingawi, N. W. & Balleine, B. W. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci 32, 1073–1081, https://doi.org/10.1523/JNEUROSCI.4806-11.2012 (2012).
- 72.
Grant, S. et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93, 12040–12045 (1996).
- 73.
Childress, A. R. et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156, 11–18, https://doi.org/10.1176/ajp.156.1.11 (1999).
- 74.
Mahler, S. V. & Berridge, K. C. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology 221, 407–426, https://doi.org/10.1007/s00213-011-2588-6 (2012).
- 75.
Koob, G. F. & Volkow, N. D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773, https://doi.org/10.1016/S2215-0366(16)00104-8 (2016).
- 76.
Haase, L., Cerf-Ducastel, B. & Murphy, C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage 44, 1008–1021, https://doi.org/10.1016/j.neuroimage.2008.09.044 (2009).
- 77.
Fotros, A. et al. Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [(1)(8)F]fallypride study in cocaine dependent participants. Neuropsychopharmacology 38, 1780–1788, https://doi.org/10.1038/npp.2013.77 (2013).
- 78.
Weiss, F. et al. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA 97, 4321–4326 (2000).
- 79.
Berglind, W. J., Case, J. M., Parker, M. P., Fuchs, R. A. & See, R. E. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience 137, 699–706, https://doi.org/10.1016/j.neuroscience.2005.08.064 (2006).
- 80.
Tuominen, L. et al. Aberrant mesolimbic dopamine-opiate interaction in obesity. NeuroImage 122, 80–86, https://doi.org/10.1016/j.neuroimage.2015.08.001 (2015).
- 81.
Schultz, W. Behavioral dopamine signals. Trends Neurosci 30, 203–210, https://doi.org/10.1016/j.tins.2007.03.007 (2007).
- 82.
Ting, A. K. R. & van der Kooy, D. The neurobiology of opiate motivation. Cold Spring Harb Perspect Med 2, https://doi.org/10.1101/cshperspect.a012096 (2012).
- 83.
Mamaligas, A. A., Cai, Y. & Ford, C. P. Nicotinic and opioid receptor regulation of striatal dopamine D2-receptor mediated transmission. Sci Rep 6, 37834, https://doi.org/10.1038/srep37834 (2016).
- 84.
Nomura, Y. et al. Age-related decline of dopamine D2/3 receptor availability measured with [C-11]raclopride in non-striatal human brain regions: Comparison of four methods. NeuroImage 41, T133–T133, https://doi.org/10.1016/j.neuroimage.2008.04.101 (2008).
- 85.
Alakurtti, K. et al. Long-term test-retest reliability of striatal and extrastriatal dopamine D2/3 receptor binding: study with [(11)C]raclopride and high-resolution PET. J Cereb Blood Flow Metab 35, 1199–1205, https://doi.org/10.1038/jcbfm.2015.53 (2015).
- 86.
Piccini, P., Pavese, N. & Brooks, D. J. Endogenous dopamine release after pharmacological challenges in Parkinson’s disease. Ann Neurol 53, 647–653, https://doi.org/10.1002/ana.10526 (2003).
- 87.
Sawamoto, N. et al. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain 131, 1294–1302, https://doi.org/10.1093/brain/awn054 (2008).
- 88.
Svensson, J. E. et al. Validity and reliability of extrastriatal [(11)C]raclopride binding quantification in the living human brain. NeuroImage, 116143, https://doi.org/10.1016/j.neuroimage.2019.116143 (2019).
- 89.
Tsukada, H. et al. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res 849, 85–96 (1999).
- 90.
Hassoun, W. et al. PET study of the [11C]raclopride binding in the striatum of the awake cat: effects of anaesthetics and role of cerebral blood flow. European journal of nuclear medicine and molecular imaging 30, 141–148, https://doi.org/10.1007/s00259-002-0904-4 (2003).
- 91.
Serafini, G., Howland, R. H., Rovedi, F., Girardi, P. & Amore, M. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 12, 444–461, https://doi.org/10.2174/1570159X12666140619204251 (2014).
- 92.
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47, 351–354 (2000).
- 93.
Browne, C. A. & Lucki, I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4, 161, https://doi.org/10.3389/fphar.2013.00161 (2013).
- 94.
Aalto, S. et al. Ketamine does not decrease striatal dopamine D2 receptor binding in man. Psychopharmacology 164, 401–406, https://doi.org/10.1007/s00213-002-1236-6 (2002).
- 95.
Hashimoto, K., Kakiuchi, T., Ohba, H., Nishiyama, S. & Tsukada, H. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur Arch Psychiatry Clin Neurosci 267, 173–176, https://doi.org/10.1007/s00406-016-0692-7 (2017).
- 96.
Alstrup, A. K. et al. Effects of anesthesia and species on the uptake or binding of radioligands in vivo in the Gottingen minipig. BioMed research international 2013, 808713, https://doi.org/10.1155/2013/808713 (2013).
- 97.
Lillethorup, T. P. et al. Longitudinal monoaminergic PET imaging of chronic proteasome inhibition in minipigs. Sci Rep 8, 15715, https://doi.org/10.1038/s41598-018-34084-5 (2018).
- 98.
Ichise, M., Toyama, H., Innis, R. B. & Carson, R. E. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab 22, 1271–1281, https://doi.org/10.1097/01.WCB.0000038000.34930.4E (2002).
- 99.
Logan, J. et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16, 834–840, https://doi.org/10.1097/00004647-199609000-00008 (1996).
- 100.
Endres, C. J., Bencherif, B., Hilton, J., Madar, I. & Frost, J. J. Quantification of brain mu-opioid receptors with [11C]carfentanil: reference-tissue methods. Nucl Med Biol 30, 177–186 (2003).
- 101.
Panksepp, J. & Bishop, P. An autoradiographic map of (3H)diprenorphine binding in rat brain: effects of social interaction. Brain Res Bull 7, 405–410 (1981).
- 102.
Landau, A. M. et al. Electroconvulsive stimulation differentially affects [(11)C]MDL100,907 binding to cortical and subcortical 5HT2A receptors in porcine brain. J Psychopharmacol, 269881119836212, https://doi.org/10.1177/0269881119836212 (2019).
- 103.
Bjarkam, C. R., Glud, A. N., Orlowski, D., Sorensen, J. C. H. & Palomero-Gallagher, N. The telencephalon of the Gottingen minipig, cytoarchitecture and cortical surface anatomy. Brain Struct Funct 222, 2093–2114, https://doi.org/10.1007/s00429-016-1327-5 (2017).
- 104.
Orlowski, D., Glud, A. N., Palomero-Gallagher, N., Sorensen, J. C. H. & Bjarkam, C. R. Online histological atlas of the Gottingen minipig brain. Heliyon 5, e01363, https://doi.org/10.1016/j.heliyon.2019.e01363 (2019).
Acknowledgements
An Aarhus University “AU Ideas Project Development Grant” to AML funded the study. We are grateful for the technical support from the staff at the Aarhus University Hospital PET Centre and the Aarhus University Farm for help with the treatment of the animals. We thank Professor Morten Kringelbach, Professor Jørgen Scheel-Kruger and Associate Professor Arne Møller for help with the initiation of these studies.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.