Erectile dysfunction (ED) is common with aging. Formerly ED treatment was offered mainly by urologists, but the approval and widespread use of phosphodiesterase inhibitors has enabled primary care clinicians to provide targeted ED treatment. Although large, multicenter clinical trials have shown efficacy and safety with these drugs, they are ineffective in 30–35% of men, may cause side-effects, and do not improve the underlying pathology. A thorough understanding of erectile physiology and causes of ED and a comprehensive treatment plan addressing all contributing factors may be more effective than pharmaceutical management and may improve aspects of psychological and physical health beyond erectile problems.
Erectile dysfunction (ED) – the inability to develop and maintain an erection firm enough for penetration – is common with aging. Approximately 40% of men aged 40 and 70% of men aged 70 have some form of ED.1 ED is associated with multiple physical conditions, may be a harbinger of metabolic or vascular disease, and affects psychosocial health. Phosphodiesterase type 5 inhibitors (PDE5i) are the most common first line treatment for ED. Large, multicenter clinical trials have shown efficacy and safety with these drugs; however, they are ineffective in 30–35% of patients,2 may cause side effects, and do not improve the underlying pathology. Comprehensive treatment for ED that focuses on all contributing factors may be more effective than pharmaceutical management and may improve aspects of psychological and physical health beyond erectile problems.
ED occurs from multifactorial, complex mechanisms involving the nervous, vascular, and endocrine systems. A basic review of anatomy and physiology of erections will provide a framework to understand pathophysiology and rationale for treatment options (Figure 1). Structure of the penis consists of two vascular tissue cylinders (corpora cavernosa) that run the length of the penile shaft along with the corpus spongiosum surrounding the urethra. Penile tissue is innervated by autonomic (sympathetic and parasympathetic) and somatic (sensory and motor) aspects of the peripheral nervous system. Sympathetic nerves arise from T11–L2 and are anti-erectile, controlling ejaculation and detumescence. Parasympathetic nerves arise from S2–S4 and are pro-erectile. Sympathetic and parasympathetic nerves merge to form cavernous nerves which enter the corpora cavernosa, corpus spongiosum, and glans penis, regulating blood flow during erection. The pudendal nerve provides sensation to the entire pelvis and motor function to all sphincters, pelvic floor, and rigidity muscles.
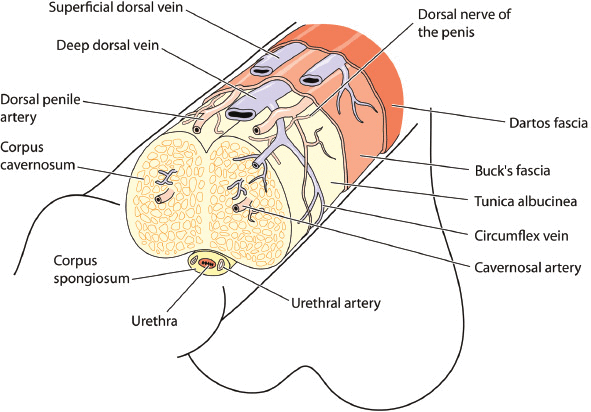
Figure 1:
Anatomy of the penis showing the major structures, blood vessels and nerves.
The internal pudendal arteries provide blood flow to the penis, branching into the bulbourethral, dorsal, and cavernosal arteries. The bulbourethral artery passes through the deep penile (Buck) fascia, supplying the bulb of the penis and penile urethra. The dorsal artery travels between the dorsal nerve and deep dorsal vein giving off circumflex branches that accompany the circumflex veins with terminal branches in the glans. The deep penile or cavernosal artery enters the corpus cavernosum at the crus and runs the length of the penile shaft, supplying the specialized helicine arteries.
Sexual stimulation triggers parasympathetic nerves to release acetylcholine. Inside the endothelial cells lining penile arteries, nitric oxide synthase (NOS) catalyzes the oxidation of L-arginine to nitric oxide (NO) and L-citrulline. NO activates guanylate cyclase in the corpora cavernosa and spongiosum, which in turn increases cyclic guanosine monophosphate (cGMP) leading to vascular smooth muscle relaxation, vasodilation, and increased blood flow. Rapid filling and expansion of the sinusoidal system causes trapping of blood by occlusion of venous plexuses and the tunica albuginea, resulting in almost total occlusion of venous outflow. Intracavernosal pressure reaches 100 mmHg at full erection. Ischiocavernous muscles compress blood-filled cavernosa as perineal muscles contract causing final pressure to reach several hundred mmHg. After ejaculation, neurotransmitter release ceases due to sympathetic nerve excitation and phosphodiesterase enzymes break down cGMP leading to detumescence and flaccidity (Figure 2).
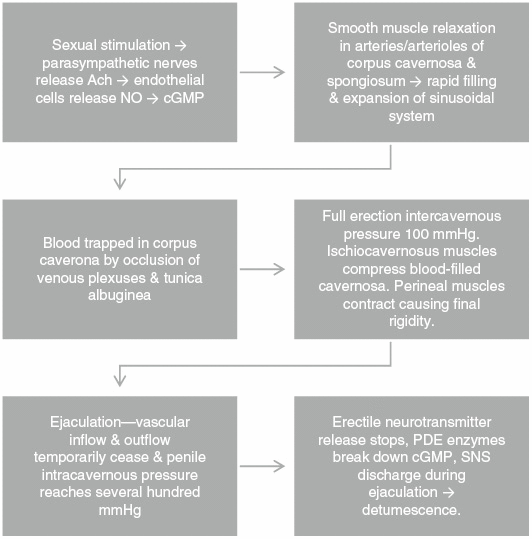
Figure 2:
Physiology of erections.
Ach, acetylcholine; cGMP, cyclic guanosine monophosphate; NO, nitric oxide; PDE, phosphodiesterase; SNS, sympathetic nervous system.
Thorough evaluation of ED requires a comprehensive history, use of validated questionnaires, physical exam, and lab work. Imaging, such as duplex doppler ultrasound, penile arteriography, and MRI should be performed by a urologist and reserved for potential surgical intervention.
HISTORY
Taking a thorough history provides the opportunity to identify all causes/contributing factors related to ED. Regarding psychosocial factors, asking about current stressors and relationship problems is imperative.
Genital self-image and sexual frequency expectations are often neglected topics when taking a sexual history, but significantly affect sexual performance. Results from the development and validation of the Male Genital Self-Image Scale (MGSIS) that assessed men ages 18–60 found that men with better genital self-image reported less ED and 20% of men were dissatisfied with their penis size.3 Men who have negative genital self-image may wonder how their penis size compares to other men. Alfred Kinsey’s data on 2500 men reported the average flaccid penis length as 1–4 inches and an average erect penis as 5–6.5 inches. Interestingly, men tended to underestimate their penis size compared to actual measurement.4
Discussing expectations about frequency of sexual activity is relevant for men with low libido, feelings of sexual inadequacy, or curiosity about their sexual frequency compared with other men. Statistics about frequency of sexual encounters is limited. One AARP survey of 1670 men and women over the age of 45 reported that 41% of men in their 50s, 24% of men in their 60s, and 15% of men in their 70s have sex at least once per week (reframing these statistics – 59% of men in their 50s, 76% in their 60s, and 85% of men in their 70s have sex less often than once per week).5 The International Society for Sexual Medicine (ISSM), reporting results from the Kinsey Institute’s 2010 National Survey of Sexual Health and Behavior, noted that just under half of married men aged 25–49 had sex a few times per month to weekly, which was the highest rate in any age category.6 Another study published by ISSM surveying men and women over age 50 reported that only 20–30% of men and women remain sexually active into their 80s.7
Pornography use is a topic that may be uncomfortable for physicians to discuss with their male patients. Although the diagnosis of pornography addiction is controversial, there is increasing evidence linking frequent pornography use with an impact on partnered sexual satisfaction, marital and relationship happiness, and sexual dysfunction including low libido and ED.8–11 Internet pornography provides limitless novelty and an on-demand video format which may condition sexual arousal, making it difficult for men to transition to real-life partners.12 No validated screening tool to identify pornography-related difficulties including ED has been developed. After asking about frequency of pornography use, healthcare providers should ask about the ability to achieve and maintain an erection during partnered sex and masturbation without pornography use or recall. If use or recall of pornographic images is needed to produce an adequate erection, pornography- induced ED may be a problem.
VALIDATED QUESTIONNAIRES
Validated questionnaires are helpful in screening for ED. The most commonly used questionnaire in the clinical setting and in published research is the 15-item International Index of Erectile Function (IIEF-15), validated in 32 languages.13 A shorter version, the IIEF-5, or the Sexual Health Inventory for Men (SHIM) questionnaire may also be useful for diagnosis and to monitor treatment effectiveness.14,15
PHYSICAL EXAM
Physical exam should include a penile and testicular exam, possibly pelvic floor muscle strength (via digital testing), blood pressure, evidence of cardiovascular disease, height, weight, and waist circumference.
WORKUP
Based on history and physical exam, laboratory testing may include comprehensive metabolic and lipid panels, fasting insulin, hemoglobin A1C, inflammatory markers such as hsCRP, total and free testosterone, thyroid testing, and, in younger men with suspected hypogonadism, luteinizing hormone and prolactin.
Causes and contributing factors for ED may overlap. These include psychosocial issues, neurological problems, excessive pornography use, endocrine disorders, medication side effects, and vascular changes.
PSYCHOGENIC
Significant stress, relationship difficulties, depression, anxiety, and post-traumatic stress disorder can all contribute to ED. Performance anxiety, first described by Masters and Johnson in 1970,16 is an inability to achieve an erection due to past experiences with ED. Sexual dysfunction, especially ED and decreased sexual desire, is significantly increased in male veterans with post-traumatic stress disorder.17 Psychogenic ED usually occurs during partnered sex with normal erectile function during masturbation. The onset may be abrupt, coinciding with stress such as job loss, death of a relative, or financial problems. Nocturnal or morning erections are often normal.
NEUROLOGICAL
The limbic system, including the amygdala; hippocampus; and dentate and cingulate gyri, is one of the oldest areas of the brain common to all mammals. This brain region regulates emotion and attempts to avoid pain and seek pleasure. Research suggests that sexually-pleasing visual stimuli activate the amygdala and hypothalamus more in men than in women.18 Input from the amygdala travels to the ventral striatum, a major portion of the basal ganglia that functions as part of the reward system. The nucleus accumbens within the ventral striatum contains a large concentration of dopaminergic neurons and is considered the brain’s pleasure center. Dopamine signaling plays a central role in sexual arousal and motivation. Activation of dopamine receptors in lumbosacral parasympathetic nerves of the spinal cord facilitates erections.19
Conditions that disrupt normal dopamine signaling or neurotransmission, or that can damage the central nervous system, for example, Parkinson’s disease, multiple sclerosis, diabetes, or stroke, can also cause ED. Cavernous nerve injury during radical prostatectomy leads to ED in more than 50% of men.20 Long-distance biking can compress the pudendal nerve and blood vessels between the saddle and pubic symphysis, limiting blood flow and oxygen to the penis.21,22 Cyclists may experience temporary ED and genital numbness; however, they may not have a greater risk for ED. Results of a recent survey of 5000 athletic men showed that cyclists were just as likely to experience ED as swimmers and runners.23
PORNOGRAPHY USE
Although pornography use may be considered socially acceptable and normal, the health risks of frequent use are not known. Internet pornography provides limitless visual novelty exemplifying the Coolidge Effect, a biological phenomenon seen in male animals when they exhibit renewed interest if introduced to different receptive sexual partners.24 This provides a possible evolutionary benefit enabling a male to fertilize multiple females. Novel sexual visual stimuli provoke stronger arousal, firmer erections, and faster ejaculation with more motile sperm and semen production.25–27
Excessive use of internet pornography can influence neuroplasticity.28 All drugs of abuse and behavioral addictions, such as Internet gaming and excessive food consumption, affect the mesolimbic dopamine pathway and nucleus accumbens.29 Novelty promotes surges of dopamine in the nucleus accumbens, triggering release of cAMP response element-binding protein (CREB). CREB regulates gene expression of dynorphin, a protein that slows dopamine release, dampening the reward system.30 This is believed to be the molecular basis of tolerance as increased amounts of the drug or behavior are required to overcome increased amounts of CREB. When abstinent, dopamine reduction promotes anhedonia, potentially setting up dependence on the drug or behavior.
In addition to CREB, DeltaFosB is released with repeated dopamine flooding of the nucleus accumbens. DeltaFosB promotes positive reinforcement of the addictive behavior by suppressing dynorphin release and increasing sensitivity to the drug or behavior. DeltaFosB persists for a relatively long time, leading some addiction specialists to refer to it as the “molecular switch for addiction.”31 This mechanism explains how repeated use of pornography, like other addictive substances, causes desensitization and dopamine receptor downregulation to occur, setting up the user for a cycle of binging, craving, and erosion of willpower.32
ENDOCRINE
Besides being necessary for development and growth of the penis and enhancing sex drive, testosterone regulates erectile physiology by several mechanisms. Testosterone promotes healthy nerve structure, integrity, and function, particularly of the cavernous nerve.33 Animal and human studies suggest that testosterone enhances nitric oxide synthase gene expression and NO production in penile arteries, necessary for vasodilation.34,35 Testosterone likely modulates PDE5 activity as evidenced by animal studies showing up-regulation of PDE5 expression with testosterone supplementation.36,37
Low levels of free and bioavailable (but not total) testosterone are associated with erectile dysfunction.38 Although the level of testosterone necessary to achieve and maintain erections is unknown, a minimum amount appears to be necessary for erectile function.39,40 Testosterone supplementation may not improve ED in all men; however, some studies have shown that testosterone therapy may be helpful and enable PDE-5 inhibitors to work better.41,42
Some authors have documented high estradiol levels in men or a high estradiol-to-testosterone ratio associated with ED.43–45 Aromatase inhibitors prevent conversion of testosterone into estradiol and may increase total and bioavailable testosterone in elderly men with mild hypogonadism while slightly lowering estradiol levels.46 Currently, there is no evidence that aromatase inhibition improves sexual function and no literature to support the use of aromatase inhibitors for hypogonadism.47 In addition, some studies have found the ratio between estradiol and testosterone is unrelated to erectile function or sexual desire.48,49
High prolactin secretion is an uncommon cause of low testosterone and ED. This can be due to a pituitary tumor (prolactinoma), marijuana, or medications such as amphetamines, H2 blockers, risperidone, SSRIs, MAO inhibitors, and some tricyclic antidepressants. Prolactin should be measured only in cases of low sexual desire, gynecomastia, and/or total testosterone levels less than 4 ng/mL (400 ng/dL).50
Both hypothyroidism and hyperthyroidism can lead to ED and ED is more prevalent in men with dysthyroidism than in controls.51 Treatment of hypothyroidism and hyperthyroidism may improve ED.52 Therefore, screening for thyroid dysfunction in men presenting with ED is recommended.
VASCULAR
Optimal vascular health is paramount to achieving and maintaining erections. Vascular dysfunction causes 70–80% of non-psychogenic ED in older men. Because atherosclerosis of coronary, carotid, cerebral, or peripheral arteries may only cause symptoms when advanced, ED may be the earliest sign of generalized vascular disease.53,54 In the Prostate Cancer and Prevention Trial, nearly 10,000 men randomized to the placebo arm were evaluated every three months and followed for ED and cardiovascular disease from 1994 to 2003. ED was as big a risk factor for future cardiovascular events as smoking and family history of heart attacks.55 The presence of ED may also be a predictor of all-cause mortality.56 ED shares the same risk factors as cardiovascular disease – high blood pressure, lack of exercise, poor diet, smoking, diabetes, and hyperlipidemia. The underlying mechanism for vascular-related ED involves endothelial dysfunction.57,58 The regulation of vasodilation is a function of nitric oxide (NO) released by endothelial cells. NO initiates production of cGMP causing smooth muscle relaxation and vasodilation of arteries in the corpus cavernosum. Endothelial dysfunction increases ED risk, regardless of clinical evidence of cardiovascular disease.59,60
MEDICATION SIDE EFFECTS
Many pharmaceutical agents can contribute to ED by affecting neurotransmitters, hormones, nerve function, or blood flow. Although not an exhaustive list, common culprits include antidepressants (especially SSRIs such as fluoxetine, sertraline, citalopram), anxiolytics, CNS depressants, and muscle relaxants (lorazepam, cyclobenzaprine). Diuretics (HCTZ, spironolactone, triamterene, furosemide) and antihypertensives and beta-blockers (clonidine, enalapril, metoprolol) also commonly contribute to ED.
It is important to develop a comprehensive treatment plan for ED, as it is likely to be more effective than using a single agent to address only symptoms.
PSYCHOSEXUAL COUNSELLING
Referral for cognitive behavioral therapy, stress management, or couple’s therapy may be appropriate for some men with ED. The causal relationship between depression and ED is unclear and likely bidirectional.61 In fact, one randomized controlled trial (RCT) of 152 men with concomitant mild-to-moderate depression and ED showed improved mood in men given sildenafil when their ED improved.62 ED caused by performance anxiety or depression is best treated with individual cognitive behavioral therapy, relationship counseling, or working with a certified sex therapist. There is also evidence that group therapy may improve erectile function. A Cochrane review of 11 clinical trials (nine of which were randomized), concluded that focused sex-group therapy was more effective than no treatment for ED. A meta-analysis of trials comparing group therapy plus sildenafil citrate versus sildenafil alone, found that men who received group therapy plus sildenafil showed significant improvement of successful intercourse and were less likely than those receiving only sildenafil to drop out. Group therapy also significantly improved ED compared to sildenafil citrate alone.63
RECONDITIONING FROM EXCESSIVE PORNOGRAPHY USE
Reversing ED attributed to frequent pornography use requires the patient to eliminate all pornography, pornography substitutes, pornography recall, and essentially all artificial sexual stimulation. This allows for reconditioning sexual arousal and erectile ability with real-life partners. Although the time to “reboot” the brain with pornography avoidance is unknown, pornography addiction expert Gary Wilson suggests clinical experience and online forums show quicker recovery for men over 50 years, suggesting that 2 months is typical.64 Younger men may need more time, possibly up to 5 months, with the theory that their Internet pornography use started at a younger age. Sexual arousal is conditioned, especially during childhood and adolescence, and may be stronger in men than in women.65–67
PHYSICAL THERAPY
Pelvic floor muscles that play a role in maintenance of erections weaken with age. Physical therapy to strengthen the bulbocavernosus and ischiocavernosus muscles and connective tissue can effectively treat ED in some patients. In one randomized, controlled study, 40 men with ED were taught to maximally retract the penis and lift their scrotum twice daily while standing, sitting, and lying down, and to tighten their pelvic floor muscles after urinating. Results were surprising – by 6 months, 40% of participants regained normal erectile function and 35% showed some improvement; 66% of the men also reported decreased dribbling after urination. A simpler technique may be to teach male patients Kegel exercise by having them stop urine midstream to identify muscles that will be needed to perform the exercise. These muscles should be contracted for 5 seconds, 10–20 times in a row, three times per day. Men may be incentivized to perform Kegel or pelvic floor strengthening exercises due to possible improvement in orgasm quality, a common side effect Arnold Kegel, MD documented several decades ago in women who performed frequent Kegels.68
VACUUM CONSTRICTION DEVICE
The vacuum constriction device (VCD), colloquially referred to as a “penis pump” was designed by Geddings Osbon in 1974.69 Osbon referred to it as a “youth equivalent device” and claimed to personally use it for 20 years without failure. The first vacuum constriction device was FDA approved for ED in 1982.
The VCD works by increasing blood flow to the penis through generating 110–225 mmHg negative pressure (manually or by battery-operated pump) and preventing venous outflow with a constriction ring. Studies suggest approximately 55–70% of men can achieve adequate erections with VCDs.70, 71 Some men report that the erection obtained from a VCD tends to be purplish, cold, or numb, and side effects include bruising of the penile shaft and trapping of ejaculate during orgasm from the constriction band. The constriction ring should not be left in place for more than 30 minutes due to risk of ischemia.
DIET, EXERCISE, AND WEIGHT LOSS
The search for aphrodisiacs to stimulate libido and improve sexual performance dates to antiquity. Indeed, the word aphrodisiac comes from the Greek goddess of love, Aphrodite, who was born from the sea and brought to shore in a scallop or oyster shell. Although oysters contain ample amounts of zinc needed for testosterone production, eating them has not been shown to improve libido or erectile ability.
Certain foods, however, do improve vascular health and may, therefore, enhance erectile function. For example, foods high in nitrates such as beets and leafy greens raise nitric oxide levels, promote normal endothelial function, and lower blood pressure.72–74 Pomegranate seeds and juice also improve endothelial function and lower blood pressure while decreasing oxidized and glycated LDL, thereby minimizing atherosclerotic plaque formation and mitigating arterial wall thickness and stiffness.75–78 High fructose corn syrup and soft drinks raise the risk of developing metabolic syndrome, atherosclerosis, diabetes, and ED.79 In addition, foods with high advanced glycation end products such as bacon, fast food hamburgers, hot dogs, cheese, pizza, and fried food, contribute to diabetes, cardiovascular disease, and ED.80–82 Rather than focusing on avoiding specific foods, it may be most beneficial for ED patients to adopt a Mediterranean diet with copious intake of vegetables, fruits, extra virgin olive oil, whole grains, nuts, and fish and moderate intake of wine. Evidence from four clinical trials suggests that the Mediterranean diet and lifestyle positively influence sexual function,83,84 as well as lowering inflammation and delaying sexual dysfunction in diabetic men.
There is little debate that exercise improves several risk factors that contribute to ED including reducing inflammation, improving endothelial function, promoting insulin sensitivity, improving lipoproteins, and enhancing visceral fat loss.85–89 A recent systematic review and meta-analysis confirms that moderate-to-vigorously intense exercise for a minimum of 8 weeks can improve ED.90
Both excessive and insufficient body fat are associated with ED. For example, the Hallym Aging Study measured body fat percentage and its relationship to ED in Korean men.91 Men with the lowest and highest body fat were more likely to have ED. Central obesity is associated with metabolic syndrome, vascular dysfunction, and low testosterone, all of which contribute to the development of ED.92,93 Fat tissue secretes more than 35 hormones and cytokines, nearly all of which promote inflammation, insulin resistance, and eventually, vascular disease.94,95 Inflammation appears to be a key player in the cause of ED. Obese men with ED have higher levels of inflammatory markers (IL-6, IL-9, IL-18, and CRP) and impaired endothelial function than obese men without ED.96
Weight loss can significantly improve erectile function. In one RCT, obese men who lost an average of 33 pounds over 2 years improved sexual performance.97 ED improved in more than 30% of the weight loss group compared to 5% of controls. The intervention group received nutritional counseling, advice to increase physical activity to approximately 3 hours per week, and monthly or bimonthly meetings. Besides an average weight loss of 15%, men in the intervention group also showed reduction in inflammatory markers IL-6 and hsCRP, improving their cardiometabolic risk profile.
BOTANICALS AND AMINO ACIDS
The use of botanicals, nutrients, and other natural therapies to boost sexual performance has increased considerably due to Internet marketing. Few natural therapies have undergone human clinical trials to support safety and efficacy. However, the following botanicals and amino acids may be helpful in treating ED, especially for men who prefer not to use PDE5i medications.
Pausinystalia yohimbe
Yohimbe is an evergreen native to central Africa that contains three alkaloids: rauwolscine, corynanthine, and yohimbine. The most active constituent of yohimbe, yohimbine, is a pharmaceutical with a well outlined mechanism of action as an antagonist of presynaptic α1 and α2-adrenergic and 5-HT(1B) receptors and partial agonist of 5-HT(1A) receptors.98 Meta-analyses suggest that yohimbine is effective for ED99,100 Yohimbine may also help with delayed ability or inability to ejaculate.101 Dosage is 15–30 mg, up to 100 mg per day. Yohimbe may be best delivered on-demand since onset is quick, within 10–15 minutes, with a 35-minute half-life. Yohimbine penetrates the central nervous system with possible side effects including tachycardia, hypertension, irritability, and anxiety. Sweating, nausea, dizziness, headache, and skin flushing are also common. As with all botanicals, many over-the-counter supplement brands may not be reliable. One study testing 49 yohimbe brands found considerable variability in the amount of yohimbine – 0 to 12.1 mg – per serving with 19 brands containing no rauwolscine and corynanthine, suggesting they were from highly processed plant extract or synthetic in origin.102
Tribulus terrestris
T. terrestris grows in Europe, Asia, Africa, and the Middle East. The root and fruit have long-term use in both Chinese and Ayurvedic medicine. Claims are frequently made that Tribulus improves testosterone production; however, clinical trials have not supported this assumption except in intravenous use in primates.103–106 Animal studies have shown that Tribulus may improve erectile function and NO production.107,108 One RCT of 180 men with mild-to-moderate ED using 500 mg of standardized T. terrestris taken three times daily reported improved libido, ED, intercourse satisfaction, and orgasm quality. No adverse effects were reported.109
Eurycoma longifolia
Eurycoma longifolia, known as Malaysian ginseng or Tongkat Ali, is a flowering plant native to Indonesia, Malaysia, Thailand, Vietnam, Laos, and India. A meta-analysis of RCTs suggest E. longifolia significantly ameliorates ED.110 In addition, a Chinese review of published studies suggests E. longifolia improves semen volume, libido, and testosterone.111 The plant may have an adaptogenic ability, and has been shown to reduce fatigue, improve well-being, lower cortisol, and increase testosterone in stressed subjects.112 Taken as a water root extract, Eurycoma appears safe without significant side effects. Suggested dosage is 200–300 mg once or twice daily, with a patented form standardized to 22% eurypeptides and 40% glycosaponins.
Epimedium spp.
Epimedium spp. grow in China and Korea, and belong to the Berberidaceae family (which also contains the well-known botanicals, Mahonia aquifolium, Hydrastis canadensis, and Berberis vulgaris). At least 50 species have been identified and are commonly known as “horny goat weed”. The presumed active ingredient, icariin, is a flavonoid that improves erectile function in animal studies via PDE5 inhibition and nitric oxide induction.113–115 – Human clinical trials on Epimedium are lacking; therefore, dosage recommendations are not available.
L-arginine
L-arginine is an amino acid, essential in conditions with increased arginase enzyme, such as diabetes and renal failure.116,117 Arginine is used by intestinal enterocytes and hepatocytes and converted into L-citrulline or L-ornithine. Variability in absorption of oral L-arginine is considerable – a 6 g dosage is approximately 68% absorbed whereas a 10 g dosage is only 20% absorbed.118,119
Nitric oxide is the byproduct of L-arginine conversion to citrulline. In addition, citrulline supplementation increases plasma arginine. Arginine may improve ED in high dosages; for example, 5000 mg improves ED, especially if urinary NO metabolites are low.120 Theoretically, L-arginine may work best if ADMA levels are elevated. Since ADMA inhibits eNOS, the endothelial enzyme needed for NO production, L-arginine supplementation may re-establish the arginine-to-ADMA ratio.121 L-arginine supplementation may be more effective for ED when combined with yohimbine or pycnogenol (pine bark of Pinus pinaster).122–124 Supplementation with L-arginine may activate herpes and increase outbreaks. In addition, an RCT published in 2013 reported 3000 mg of L-arginine taken 3 times per day increased mortality risk in patients with recent MI.125
PHARMACOLOGICAL THERAPIES
The main pharmacological approaches for ED are PDE5 inhibitors, apomorphine, and intracavernosal injection therapies.
PDE5 inhibitors
Phosphodiesterase type 5 inhibitors (PDE5i) are the most common drug treatment for ED. Their mechanism of action is well known, inhibiting the phosphodiesterase type 5 enzyme which breaks down cGMP, effectively prolonging NO activity in penile arteries. Medications in this class differ in their selectivities for the 11 different PDE isoenzymes. This cross-reactivity with PDE isoenzymes is especially found in vascular, visceral, and pulmonary smooth muscle and contributes to side effects.126 For example, vardenafil and sildenafil are three and seven times more likely to bind to PDE6, an enzyme in the retina that transfers light into nerve impulses. Inhibition of this enzyme causes color perception disturbances known as “chromatopsia.”
Four PDE5i drugs have been FDA approved – sildenafil in 1998, vardenafil and tadalafil in 2003, and avanafil in 2012. No head-to-head trials comparing effectiveness among these drugs have been performed. These medications differ in onset, duration of action, and side-effects.127 The onset of sildenafil and vardenafil is 30–60 minutes with a duration of 10–12 hours. Tadalafil’s onset is 15–30 minutes depending on dosage, with the longest duration of 36 hours. Avanafil has the shortest onset, within 15 minutes, and duration, approximately 6 hours.
PDE5i use may lower prostate cancer risk, possibly because men who use them may ejaculate more frequently, which protects against prostate cancer.128,129 Long-term sildenafil citrate (Viagra) use is associated with a slightly increased risk for melanoma.130 Most common side effects of PDE5i include headache in up to 20% of men, flushing in up to 15%, and dyspepsia and nasal congestion in up to 10%. Although uncommon, dizziness and priapism may occur. All PDE5i are contraindicated in men taking nitrates.
Apomorphine
Apomorphine has been in use since 1869 for Parkinson’s disease, and has been studied for its effect on erectile function. Although derived from morphine, apomorphine does not contain morphine or bind to opioid receptors. It does have high affinity for dopamine receptors and the mechanism for improving sex drive and erections is likely via moderate affinity for D2 receptors in the hypothalamus and limbic system.131 In human phase II and III clinical trials involving 5000 men, 3–4 mg of sublingual apomorphine produced erections firm enough for penetration within 10–25 minutes, with approximately 20–25% improvement over placebo.132,133 The only patented pharmaceutical apomorphine in the US is injectable Apokyn, FDA-approved for advancing Parkinson’s disease; however, apomorphine can be compounded as a sublingual lozenge and can be combined with a PDE5i. Dosages of 2–3 mg may be as effective as those of 4–6 mg without side effects such as nausea, headache, or dizziness. Apomorphine should not be used concomitantly with ondansetron hydrochloride, a frequently prescribed antiemetic, due to possible hypotension.
Intracavernosal injections
Introduced in 1983, intracavernosal injections modulate endothelial function and are very effective even in men with severe ED; alprostadil produces erections in up to 93% of men, with effectiveness of bi-, tri-, and quad-mix of up to 97.6%.134–136 Alprostadil, 20 or 40 μg of prostaglandin E1, is the only FDA-approved patented intracavernosal injection, whereas two other medications, phentolamine and papaverine, can be added to PGE1 in a compounded formula. The most common side effect of PGE1 alone is pain in 48.5% of men. Bi-mix, which often contains 0.5–3.0 mg of phentolamine and 30 mg papaverine, does not cause pain but may not be as effective. Tri-mix, usually containing 5–10 μg (and up to 40 μg) of PGE1, 0.5–1.0 mg phentolamine, and 15–30 mg papaverine, reduces pain likelihood to 2.9%. The addition of 0.15 mg of atropine in quad-mix, reserved for men in whom tri-mix is ineffective, significantly ameliorates pain. The amount of PGE1, bi-, tri-, or quad-mix needed varies from 0.1 to 0.3 mL. Empiric (using PGE1 only regardless of ED etiology or severity, with dosage or formula adjustments made based on patient results) and risk-based (using bi-mix, tri-mix, or high-dose tri-mix based on an algorithm factoring ED etiology and number of ED risk factors) approaches to dosing appear to be similar regarding effectiveness and complication and satisfaction rates.137 Side effects include pain at injection site, priapism, and development of scar tissue or Peyronie’s disease. For an excellent article reviewing effectiveness, dosages, and side effects of intracavernosal injections, see Medscape’s summary “Intracavernosal Injection Algorithm” by Jeffrey Albaugh.138
LOW-INTENSITY EXTRACORPOREAL SHOCKWAVE THERAPY
Low intensity extracorporeal shockwave (LI-ESW) originated in the 1990s when ultrasound was shown to induce angiogenesis in rat wounds.139 LI-ESW uses shock waves, a type of acoustic wave that carries energy and induces biological reactions when applied to target tissue.140 The procedure varies in terms of energy flux density, frequency (number of pulses per second in Hz), and total number of pulses delivered. The mechanism of action for ED improvement appears to be regeneration of penile neuronal nitric oxide (nNOS) positive nerves, improved nitric oxide release, and endothelial and vascular smooth muscle cell repair via recruitment of mesenchymal stem cells.141 The treatment may also activate local penile progenitor cells.142 Currently, LI-ESW is not FDA-approved for ED; however, many clinical trials have been performed with a good safety profile and varying success.
The first ED pilot study published in 2010 used six LI-ESW sessions in 20 men who were non-responders to PDE5 inhibitors (PDE5i). Results showed improved erectile function, duration of erections, and penile rigidity in 1 month. Improvements were reported for up to 6 months of follow up.143 Several RCTs have reported positive outcomes using LI-ESW. In one trial involving 67 men with ED who responded to PDE5i, the treatment arm received 12 sessions with improved erectile function and penile hemodynamics seen in approximately 50% of men without the use of their PDE5i.144 In a similar RCT in India including 135 PDE5i responders treated with 12 sessions, 78% of treated men were able to achieve erections firm enough for penetration without medication at 1 month.145 Although these results were sustained at 1 year follow-up, there was a very high dropout rate including 58% of the sham and 42% of the treatment arms.
Men who do not respond to PDE5i may become responders after LI-ESW treatment. In an open-label, single-arm prospective study of 29 men non-responsive to PDE5i, 12 treatments resulted in 72% of men able to achieve erections firm enough for penetration with a PDE5i.146 In a more recent RCT including 58 PDE5i non-responders, 54% responded to PDE5i after 1 month of LI-ESW therapy compared to 0% in the sham group.147
Sustained improvement seen in longer follow-up studies suggests that following treatment, some men may reverse the underlying pathology causing their ED or that LI-ESW may provide some degree of penile rehabilitation. In one RCT with 6 month follow-up of 112 men, all of whom received five treatment sessions since the placebo arm received active treatment at 10 weeks, at 6 months, approximately 20% of the initial treatment arm and 23% of the treated initial placebo group were still able to have intercourse without medication.148 Another 1 year follow-up of 50 older men (average age 65 years) with vascular risk factors including diabetes, hypertension, dyslipidemia, and coronary artery disease found a 60% sustained improvement in ED severity and self-reported erection quality.149
The number of LI-ESW treatments for ideal outcome and how long treatment remains effective is not known. A recent RCT performed on 126 men in a Danish hospital compared men who received five versus ten sessions at 6 and 12 months; treatment was approximately 38% effective in both groups, suggesting that additional sessions may not improve outcome.150,151 In a 2 year follow-up of an open-label trial of 156 men, 63% improved at 4 weeks with 53% effectiveness sustained at 2 years.152 Not surprisingly, men with severe ED had earlier failure. All patients with diabetes and severe ED lost the effect, whereas 76% of men with mild ED and without diabetes preserved effectiveness.
The number of studies using LI-ESWT for ED has increased dramatically in recent years. A narrative review of published literature performed in 2013 reported that 60–75% of PDE5i responders could achieve erections firm enough for penetration without medication and 72% of PDE5i non-responders became responders.153 Recent meta-analyses reviewing 14 studies including 7 RCTs found that LI-ESW therapy is safe and effective, with results lasting at least 3 months.154 Men with mild or moderate ED appear to have a better response than men with severe ED, with energy flux density, number of shock waves delivered, and duration of treatment closely correlated with outcome.
PRP AND STEM CELL INJECTION
Injection of platelet rich plasma (PRP) or mesenchymal stem cells into the corpus cavernosum holds promise to improve ED and restore penile artery and nerve function. To prepare PRP, platelets from anti-coagulated blood are spun in a centrifuge and concentrated. PRP contains more than 300 bioactive proteins, growth factors, and adhesion molecules that can improve tissue healing and promote nerve and vascular tissue regeneration.155–157 Since penile vasculature is the most endothelial-rich region of a man’s body and blood flow in the flaccid penis is slower compared with systemic circulation (allowing for better retention), theoretically, PRP may provide benefits in penile tissue similar to improvements seen in orthopedic injuries.
Few safety and feasibility pilot studies using PRP for ED have been published. One human trial conducted in Italy evaluated 9 men with ED who received PRP in addition to vacuum therapy. Mild improvement was seen with the only minor adverse effect of mild pain and bruising at the injection site.158
Reduced levels of circulating endothelial progenitor cells (EPCs), a type of stem cell necessary for regeneration of the vascular endothelial lining, is an independent risk factor for ED.159 EPCs are decreased with chronic inflammation seen in diabetes, hypercholesterolemia, obesity, cardiovascular disease, and cigarette smoking.160 Animal studies suggest testosterone stimulates EPC mobilization from bone marrow and improves angiogenesis.161,162 Two reviews of published animal studies involving intracavernosal injections of stem cells derived from bone marrow, adipose tissue, and skeletal muscle reported favorable outcomes on endothelial, smooth muscle, and nerve function in penile tissue.163,164
PRP and stem cell therapy may help men with vascular and nerve injuries from diabetes or radical prostatectomy. A few human pilot studies suggest the procedure may be safe and effective. One study involved 11 men who underwent radical prostatectomy due to prostate cancer, with resulting ED non-responsive to PDE5i.165 Autologous adipose-derived stem cells injected into the corpus cavernosum recovered erectile function in eight of the eleven men without adverse effects. In another pilot study using non-autologous human umbilical cord stem cells, six out of seven older men with diabetes and ED non-responsive to PDE5i, regained morning erections by the third month, and two out of seven were still able to achieve an erection with PDE5i after 6 months. Interestingly, blood glucose decreased 2 weeks after injection, with hemoglobin A1C levels improved for up to 4 months, and no adverse effects were seen. Positive results were also seen in a human pilot study involving 12 men with severe post-radical prostatectomy ED non-responsive to alprostadil, PDE5i, or vacuum device.166 This phase I trial used escalating doses of autologous bone marrow-derived stem cells. Higher doses were more effective with nine out of twelve men able to achieve erections firm enough for penetration with PDE5i; benefits were sustained at one year without adverse effects.
While PRP and stem cell injections are considered experimental and lack FDA-approval and insurance coverage, men may seek out practitioners who perform these procedures. Possible adverse effects include infection and development of scar tissue or Peyronie’s disease. Theoretically, stem cells may also increase tumorigenesis, although the extent of this risk is unknown.167
Erectile dysfunction is a common medical problem with several possible, often overlapping contributing factors and causes. Careful and thorough history taking, use of validated questionnaires, physical exam, and lab work up is necessary to develop a plan to ameliorate symptoms while addressing underlying causes. Treatment options include diet and lifestyle modifications, physical therapy exercise, the use of vacuum constriction devices, botanical and amino acid dietary supplements, medications, low intensity extracorporeal shockwave therapy, and possibly platelet rich plasma or stem cell intracavernosal injections.
- Perform thorough history, questionnaire documentation, exam, relevant lab work
- Discuss causes/contributing factors:
- Psychogenic
- Neurological
- Excessive pornography use
- Endocrine
- Medication side effects
- Vascular changes
- Review relevant treatment options:
- Psychosexual counseling
- Pornography abstinence
- Physical therapy (Kegel’s) or use of vacuum constriction device
- Dietary changes
- Exercise recommendation
- Weight loss options
- Herbal remedies and amino acids
- Medications:
- PDE5i: different onsets, duration of action, and side-effects
- May or may not be covered by insurance, can be expensive
- Apomorphine, not FDA-approved for ED, must be compounded
- PDE5i: different onsets, duration of action, and side-effects
- Low-intensity extracorporeal shockwave
- Not FDA-approved for ED and lack of insurance coverage
- Safety data are good with most studies showing positive results
- May need repeated treatments
- PRP and stem cell injections
- Not recommended due to lack of randomized trials and possible safety concerns
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study.
- McMahon CN, Smith CJ, Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail.
- Herbenick D, Schick V, Reece M, et al. The development and validation of the Male Genital Self-Image Scale: results from a nationally representative probability sample of men in the United States.
- Gebhard P, Johnson A.
- Fisher L, Anderson G, Chapagain M, et al.
- Schick V, Herbenick D, Reece M, et al. Sexual behaviors, condom use, and sexual health of Americans over 50: implications for sexual health promotion for older adults.
- Berger J, Doan A, Kehoe J, et al. PD69-12 survey of sexual function and pornography.
- Zillmann D, Bryant J. Pornography’s impact on sexual satisfaction 1.
- Sun C, Bridges A, Johnson JA, et al. Pornography and the male sexual script: an analysis of consumption and sexual relations.
- Poulsen FO, Busby DM, Galovan AM. Pornography use: who uses it and how it is associated with couple outcomes.
- Perry SL. Does viewing pornography reduce marital quality over time? Evidence from longitudinal data.
- Park B, Wilson G, Berger J, et al. Is Internet pornography causing sexual dysfunctions? A review with clinical reports.
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction.
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Dysfunction (IIEF-5) as a diagnostic tool for erectile dysfunction.
- Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience.
- Bruce T, Barlow D. The nature and role of performance anxiety in sexual dysfunction. In: Leitenberg H, ed.
- Bentsen I, Giraldi A, Kristensen E, et al. Systematic review of sexual dysfunction among veterans with post-traumatic stress disorder.
- Hamann S, Herman RA, Nolan CL, et al. Men and women differ in amygdala response to visual sexual stimuli.
- Simonsen U, Comerma-Steffensen S, Andersson KE. Modulation of dopaminergic pathways to treat erectile dysfunction.
- Campbell J, Burnett A. Neuroprotective and nerve regenerative approaches for treatment of erectile dysfunction after cavernous nerve injury.
- Ricchiuti VS, Haas CA, Seftel AD, et al. Pudendal nerve injury associated with avid bicycling.
- Oberpenning F, Roth S, Leusmann DB, et al. The Alcock syndrome: temporary penile insensitivity due to compression of the pudendal nerve within the Alcock canal.
- Awad MA, Gaither TW, Murphy GP, et al. Cycling, and male sexual and urinary function: results from a large, multinational, cross-sectional study.
- Ventura-Aquino E, Fernandez-Guasti A, Paredes R. Hormones and the Coolidge effect.
- Koukounas E, Over R. Allocation of attentional resources during habituation and dishabituation of male sexual arousal.
- Kim SC, Bang JH, Hyun JS, et al. Changes in erectile response to repeated audiovisual sexual stimulation.
- Joseph P, Sharma R, Agarwal A, et al. Men ejaculate larger volumes of semen, more motile sperm, and more quickly when exposed to images of novel women.
- Volkow ND, Baler D. Addiction science: uncovering neurobiological complexity.
- Love T, Laier C, Brand M, et al. Neuroscience of Internet pornography addiction: a review and update.
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction.
- Pitchers KK, Frohmader KS, Vialou V, et al. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator.
- Traish A, Goldstein I, Kim N. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction.
- Park KH, Kim SW, Kim KD, et al. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum.
- Mikhail N. Does testosterone have a role in erectile function?
- Morelli A, Filippi S, Mancina R, et al. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa.
- Zhang XH, Morelli A, Luconi M, et al. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum.
- Liao M, Huang X, Gao Y, et al. Testosterone is associated with erectile dysfunction: a cross-sectional study in Chinese men.
- Buena F, Swerdloff RS, Steiner BS, et al. Sexual function does not change when serum testosterone levels are pharmacologically varied in the normal male range.
- Armagan A, Kim NN, Goldstein I, et al. Dose-response relationship between testosterone and erectile function: evidence for the existence of a critical threshold.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis.
- Aversa A, Isidori AM, Spera G, et al. Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunction.
- Mancini A, Milardi D, Bianchi A, et al. Increased estradiol levels in venous occlusive disorder: a possible functional mechanism of venous leakage.
- Wu F, Chen T, Mao S, et al. Levels of estradiol and testosterone are altered in Chinese men with sexual dysfunction.
- Srilatha B, Adaikan PG, Chong YS. Relevance of oestradiol-testosterone balance in erectile dysfunction patients’ prognosis.
- Leder BZ, Rohrer JL, Rubin SD, et al. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels.
- Tan RBW, Guay AT, Hellstrom WJG. Clinical use of aromatase inhibitors in adult males.
- Dastello-Porcar AM, Martinez-Jabaloyas JM. Testosteorne/estradiol ratio, is it useful in the diagnosis of erectile dysfunction and low sexual desire?
- Gades NM, Jacobson DJ, McGree ME, et al. The associations between serum sex hormones, erectile function, and sex drive: the Olmsted Country Study of Urinary Symptoms and Health Status among Men.
- Buvat J, Lemaire A. Endocrine screening in 1,022 men with erectile dysfunction: clinical significance and cost-effective strategy.
- Gabrielson AT, Sartor RA, Hellstrom WJG. The impact of thyroid disease on sexual dysfunction in men and women.
- Krassas GE, Tziomalos K, Papadopoulou F, et al. Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat?
- Cheitlin M. Erectile dysfunction: the earliest sign of generalized vascular disease?
- Billups KL. Erectile dysfunction as an early sign of cardiovascular disease.
- Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease.
- Böhm M, Baumhäkel M, Teo K, et al. Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ON TARGET/TRANSCEND) Trials.
- Guay AT. ED2: erectile dysfunction = endothelial dysfunction.
- Aversa A, Bruzziches R, Francomano D, et al. Endothelial dysfunction and erectile dysfunction in the aging man.
- Kaya C, Uslu Z, Karaman I. Is endothelial function impaired in erectile dysfunction patients?
- Kaiser DR, Billups K, Mason C, et al. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease.
- Seidman SN, Roose SP. The relationship between depression and erectile dysfunction.
- Seidman SN, Roose SP, Menza MA, et al. Treatment of erectile dysfunction in men with depressive symptoms: results of a placebo-controlled trial with sildenafil citrate.
- Melnick T, Soares BG, Nasselo AG. Psychosocial interventions for erectile dysfunction.
- Wilson G.
- Brom M, Both S, Laan E, et al. The role of conditioning, learning and dopamine in sexual behavior: a narrative review of animal and human studies.
- Klucken T, Schweckendiek J, Merz CJ, et al. Neural activations of the acquisition of conditioned sexual arousal: effects of contingency awareness and sex.
- Griffee K, O’Keefe S, Beard K, et al. Human sexual development is subject to critical period learning: implications for sexual addiction, sexual therapy, and for child rearing.
- Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles.
- Hoyland K, Vasdev N, Adshead J. The use of vacuum erection devices in erectile dysfunction after radical prostatectomy.
- Vrijhof HJ, Delaere KP. Vacuum constriction devices in erectile dysfunction: acceptance and effectiveness in patients with impotence of organic or mixed aetiology.
- Kolettis PN, Lakin MM, Montague DK, et al. Efficacy of the vacuum constriction device in patients with corporeal venous occlusive dysfunction.
- Siervo M, Lara J, Ogbonmwan I, et al. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis.
- Hord N, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits.
- Hobbs DA, George TW, Lovegrove JA. The effects of dietary nitrate blood pressure and endothelial function: a review of human intervention studies.
- Fuhrman B, Volkova N, Aviram M. Pomegranate juice inhibits oxidized LDL uptake and cholesterol biosynthesis in macrophages.
- Aviram M, Rosenblat M, Gaitini D, et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation.
- Ignarro LJ, Byrns RE, Sumi D, et al. Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide.
- Stowe CB. The effects of pomegranate juice consumption on blood pressure and cardiovascular health.
- Adamowicz J, Drewa T. Is there a link between soft drinks and erectile dysfunction?
- Neves D. Advanced glycation end-products: a common pathway in diabetes and age-related erectile dysfunction.
- Uribarri J, del Castillo MD, de la Maza MP, et al. Dietary advanced glycation end products and their role in health and disease.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet.
- Maiorino MI, Bellastella G, Chiodini P, et al. Primary prevention of sexual dysfunction with Mediterranean diet in type 2 diabetes: the MÈDITA randomized trial.
- Di Francesco S, Tenaglia R. Mediterranean diet and erectile dysfunction: a current perspective.
- Slentz CA, Houmard JA, Johnson JL, et al. Inactivity, exercise training and detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of exercise intensity and amount.
- Di Francescomarino S, Sciartilli A, Di Valerio, et al. The effect of physical exercise on endothelial function.
- Walther C, Gielen S, Hambrecht R. The effect of exercise training on endothelial function in cardiovascular disease in humans.
- Fuchsjager-Mayrl G, Pleiner J, Wiesinger GF, et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes.
- Vina J, Sanchis-Gomar F, Martinez-Bellow V, et al. Exercise acts a drug; the pharmacological benefits of exercise.
- Silva A, Sousa N, Azevedo LF, et al. Physical activity and exercise for erectile dysfunction: systematic review and meta-analysis.
- Cho YG, Song HJ, Lee SK, et al. The relationship between body fat mass and erectile dysfunction in Korean men: Hallym Aging Study.
- Diaz-Arjonilla M, Schwarcz M, Swerdloff RS, et al. Obesity, low testosterone levels and erectile dysfunction.
- Kapoor D, Clarke S, Channer KS, et al. Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes.
- Fantuzzi G. Adipose tissue, adipokines, and inflammation.
- Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease.
- Giugliano F. Erectile dysfunction associates with endothelial dysfunction and raised proinflammatory cytokine levels in obese men.
- Evans M. Lose weight to lose erectile dysfunction.
- Millan MJ, Newman-Tancredi A, Audinot V, et al. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states.
- Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials.
- Carey MP, Johnson BT. Effectiveness of yohimbine in the treatment of erectile disorder: four meta-analytic integrations.
- Adeniyi AA, Brindley GS, Pryor JP, et al. Yohimbine in the treatment of orgasmic dysfunction.
- Cohen PA, Wang YH, Maller G, et al. Pharmaceutical quantities of yohimbine found in dietary supplements in the USA.
- Neychev V, Mitev V. Pro-sexual and androgen enhancing effects of Tribulus terrestris L.: Fact or fiction?
- Gauthaman K, Ganesan AP. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction – an evaluation using primates, rabbits and rat.
- Qureshi A, Naughton DP, Petroczi A. A systematic review on the herbal extract Tribulus terrestris and the roots of its putative aphrodisiac and performance enhancing effect.
- Neychev VK, Mitev Vi. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men.
- Gauthaman K, Ganesan AP, Prasad RN. Sexual effects of punturevine (Tribulus terrestris) extract (protodioscin): an evaluation using a rat model.
- Adaikan PG, Gauthaman K, Prasad RN, et al. Proerectile pharmacological effects of Tribulus terrestris extract on the rabbit corpus cavernosum.
- Kamenov Z, Fileva S, Kalinov K, et al. Evaluation of the efficacy and safety of Tribulus terrestris in male sexual dysfunction – a prospective, randomized, double-blind, placebo-controlled clinical trial.
- Kotirum S, Ismail SB, Chaiyakunapruk N. Efficacy of Tongkat Ali (Eurycoma longifolia) on erectile function improvement: systematic review and meta-analysis of randomized controlled trials.
- Thu HE, Mohamed IN, Hussain Z, et al. Eurycoma longifolia as a potential adoptogen of male sexual health: a systematic review on clinical studies.
- Talbott SM, Talbott J, George A, et al. Effect of Tongkat Ali on stress hormones and psychological mood state in moderately stressed subjects.
- Dell’Agli M, Galli GV, Dal Cero, et al. Potent inhibition of human phosphodiesterase-5 by icariin derivatives.
- Ning H, Xin ZC, Lin G, et al. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells.
- Jiang Z, Hu B, Wang J, et al. Effect of icariin on cyclic GMP levels and on the mRNA expression of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in penile cavernosum.
- Romero M, Platt DH, Tawfik HE, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity.
- Schramm L, La M, Heidbreder E, et al. L-arginine deficiency and supplementation in experimental acute renal failure and in human kidney transplantation.
- Curis E, Nicolis I, Moinard C, et al. Almost all about citrulline in mammals.
- Bode-Böger SM, Böger RH, Galland A, et al. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship.
- Chen J, Wollman Y, Chernichovsky T, et al. Effect of oral admnistratino of high-dose nitric oxide donor L-arginine in men with organic erectile dysfunction: results of a double-blind, randomized, placebo-controlled study.
- Bode-Böger SM, Scalera F, Ignarro LJ. The L-arginine paradox: importance of the L-arginine/asymmetrical dimethylarginine ratio.
- Lebret T, Hervé JM, Gorny P, et al. Efficacy and safety of a novel combination of L-arginine glutamate and yohimbine hydrochloride: a new oral therapy for erectile dysfunction.
- Akhondzadeh S, Amiri A, Bagheri A. Efficacy and safety of oral combination of yohimbine and L-arginine (SX) for the treatment of erectile dysfunction: a multicenter, randomized, double blind, placebo-controlled clinical trial.
- Stanislavov R, Nikolova V. Treatment of erectile dysfunction with pycnogenol and L-arginine.
- Schulman SP, Becker LC, Kass DA, L-arginine therapy in acute myocardial infarction: the vascular interaction with age in myocardial infarction (VINTAGE MI) randomized clinical trial.
- Saenz de Tejada I, Angulo J, Cuevas P, et al. Comparative selectivity profiles of tadalafil, sildenafil and vardenafil using an in vitro phosphodiesterase activity assay.
- Evans J, Hill S. A comparison of the available phosphodiesterase-5 inhibitors in the treatment of erectile dysfunction: a focus on avanafil.
- Chavez A, Coffield KS, Rajab MH, Jo C. Incidence rate of prostate cancer in men treated for erectile dysfunction with phosphodiesterase type 5 inhibitors: retrospective analysis.
- Rider JR, Wilson KM, Sinnott JA, et al. Ejaculation frequency and risk of prostate cancer: updated results with an additional decade of follow-up.
- Tang H, Wu W, Fu S, et al. Phosphodiesterase type 5 inhibitors and risk of melanoma: A meta-analysis.
- Chen KK, Chan JY, Chang LS. Dopaminergic neurotransmission at the paraventricular nucleus of hypothalamus in central regulation of penile erection in the rat.
- Altwein JE, Keuler FU. Oral treatment of erectile dysfunction with apomorphine SL.
- Heaton JP. Key issues from the clinical trials of apomorphine SL.
- Linet OI, Ogring FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction.
- Porst H, Buvat J, Meuleman E, et al. Intracavernous alprostadil alfadex – an effective and well tolerated treatment for erectile dysfunction. Results of a long-term European study.
- Baniel J, Israilov S, Engelstein D, et al. Three-year outcome of a progressive treatment program for erectile dysfunction with intracavernous injections of vasoactive drugs.
- Bernie HL, Segal R, Le B, et al. An empirical vs risk-based approach algorithm to intracavernosal injection therapy: a prospective study.
- Albaugh J. Intracavernosal injection algorithm.
- Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis.
- Lu Z, Lin G, Reed-Maldonado A, et al. Low-intensity extracorporeal shock wave treatment improves erectile function: a systematic review and meta-analysis.
- Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model.
- Lin G, Reed-Maldonado AB, Wang B, et al. In situ activation of penile progenitor cells with low-intensity extracorporeal shockwave therapy.
- Vardi Y, Appel B, Jacob G, et al. Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction.
- Vardi Y, Appel B, Kilchevsky A, et al. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study.
- Srini VS, Reddy RK, Shultz T, et al. Low intensity extracorporeal shockwave therapy for erectile dysfunction: a study in an Indian population.
- Gruenwald I, Appel B, Vardi Y. Low-intensity extracorporeal shock wave therapy – a novel effective treatment for erectile dysfunction in severe ED patients who respond poorly to PDE5 inhibitor therapy.
- Kitrey ND, Gruenwald I, Appel B, et al. Penile low intensity shock wave treatment is able to shift PDE5i nonresponders to responders: a double-blind, sham controlled study.
- Olsen AB, Persiani M, Boie S, et al. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study.
- Bechara A, Casabé A, De Bonis W, et al. Twelve-month efficacy and safety of low-intensity shockwave therapy for erectile dysfunction in patients who do not respond to phosphodiesterase type 5 inhibitors.
- Fojecki GL, Tiessen S, Osther PJ. Effect of low-energy linear shockwave therapy on erectile dysfunction-a double-blinded, sham-controlled, randomized clinical trial.
- Fojecki GL, Tiessen S, Osther PJ. Effect of linear low-intensity extracorporeal shockwave therapy for erectile dysfunction – 12-month follow up of a randomized, double-blinded, sham-controlled study.
- Kitrey ND, Vardi Y, Appel B, et al. Low intensity shock wave treatment for erectile dysfunction – how long does the effect last?
- Gruenwald I, Kitrey ND, Appel B, et al. Low-intensity extracorporeal shock wave therapy in vascular disease and erectile dysfunction: theory and outcomes.
- Clavijo R, Kohn TP, Kohn JR, et al. Effects of low-intensity extracorporeal shockwave therapy on erectile dysfunction: a systematic review and meta-analysis.
- Pavlovic V, Ciric M, Jovanovic V, et al. Platelet Rich Plasma: a short overview of certain bioactive components.
- Coppinger JA, Cagney G, Toomey S, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions.
- Yu W, Wang J, Yin J. Platelet-rich plasma: a promising product for treatment of peripheral nerve regeneration after nerve injury.
- Banno JJ, Kinnick TR, Roy L, et al. The efficacy of platelet-rich plasma (PRP) as a supplemental therapy for the treatment of erectile dysfunction (ED): initial outcomes.
- Baumhäkel M, Werner N, Bohm M, et al. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease.
- Ichim TE, Warbington T, Cristea O, et al. Intracavernous administration of bone marrow mononuclear cells: a new method of treating erectile dysfunction?
- Franck-Lissbrant I, Häggström S, Damber JE, et al. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats.
- Sieveking DP, Chow RW, Ng MK. Androgens, angiogenesis and cardiovascular regeneration.
- Mingchao Li, Ruan Y, Wang T, et al. Stem cell therapy for diabetic erectile dysfunction in rats: a meta-analysis.
- Lin CS, Xin ZC, Wang Z, et al. Stem cell therapy for erectile dysfunction: a critical review.
- Haahr MK, Jensen CH, Toyserkani NM, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial.
- Yiou R, Hamidou L, Birebent B, et al. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: an open dose-escalation pilot study.
- Mousavinejad M, Andrews P, Shoraki E. Current biosafety considerations in stem cell therapy.