Abstract
Problems arising from hypersexual behavior are often seen in clinical settings. We aimed to extend the knowledge about the clinical characteristics of individuals with hypersexual disorder (HD). A group of people who fulfilled the proposed diagnostic criteria for HD (men with HD, n = 50) was compared to a group of healthy controls (n = 40). We investigated differences in sociodemographic, neurodevelopmental, and family factors based on self-report questionnaires and clinical interviews. Men with HD reported elevated rates of sexual activity, paraphilias, consumption of child abusive images, and sexual coercive behavior compared to healthy controls. Moreover, rates of affective disorders, attachment difficulties, impulsivity, and dysfunctional emotion regulation strategies were higher in men with HD. Men with HD seem to have experienced various forms of adverse childhood experiences, but there were no further differences in sociodemographic, neurodevelopmental factors, and family factors. Regression analyses indicated that attachment-related avoidance and early onset of masturbation differentiated between men with HD and healthy controls. In conclusion, men with HD appear to have the same neurodevelopment, intelligence levels, sociodemographic background, and family factors compared to healthy controls, but they report different and adverse experiences in childhood, problematic sexual behavior, and psychological difficulties.
KEYWORDS: comorbidities; hypersexuality; phenomenology; sexual addiction; sexual compulsivity
- PMID: 30704084
- DOI: 10.3390/jcm8020157
1. Introduction
2. Experimental Section
2.1. Recruitment
2.1.1. Hypersexual Disorder Group
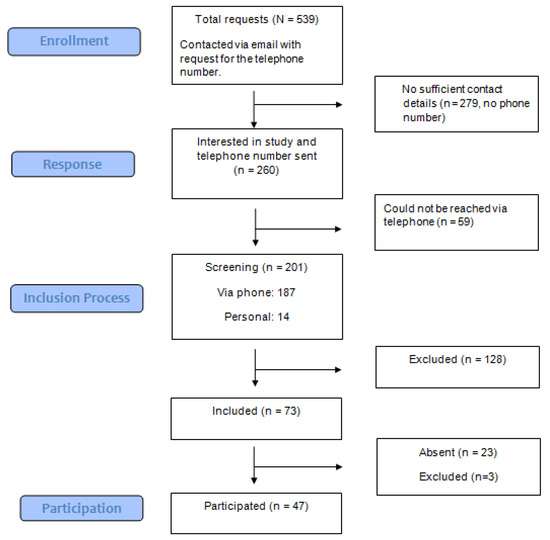
2.1.2. Healthy Controls
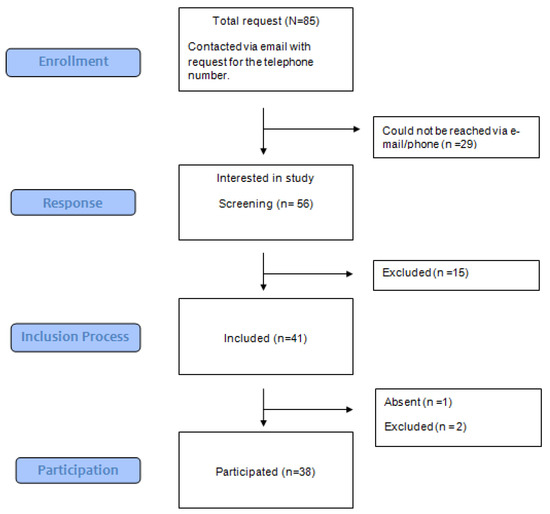
2.1.3. Exclusion Criteria
2.2. Measures




2.2.1. Sociodemographic, Neurodevelopmental, and Family Factors
2.2.2. Sexual Characteristics
2.2.3. Psychological Characteristics and Comorbidities
2.2.4. Logistic Regression Analysis
2.3. Data Analysis
3. Results
3.1. Sociodemographic, Neurodevelopmental, and Family Factors
3.2. Sexual Characteristics
3.3. Psychological Characteristics and Comorbidities
3.4. Logistic Regression Analysis
4. Discussion
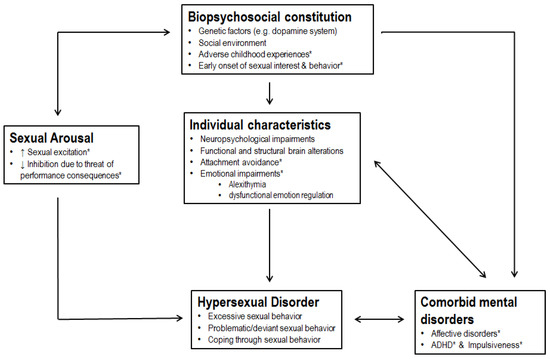
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derbyshire, K.L.; Grant, J.E. Compulsive sexual behavior: A review of the literature. J. Behav. Addict. 2015, 4, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.W.; Reid, R.C.; Parhami, I. Behavioral addictions. Where to draw the lines? Psychiatr. Clin. N. Am. 2012, 35, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Kafka, M.P. Hypersexual Disorder: A Proposed Diagnosis for DSM-V. Arch. Sex. Behav. 2010, 39, 377–400. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 089042554X. [Google Scholar]
- Kafka, M.P. What happened to hypersexual disorder? Arch. Sex. Behav. 2014, 43, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Piquet-Pessôa, M.; Ferreira, G.M.; Melca, I.A.; Fontenelle, L.F. DSM-5 and the decision not to include sex, shopping or stealing as addictions. Curr. Addict. Reports 2014, 1, 172–176. [Google Scholar] [CrossRef]
- Grant, J.E.; Atmaca, M.; Fineberg, N.A.; Fontenelle, L.F.; Matsunaga, H.; Janardhan Reddy, Y.C.; Simpson, H.B.; Thomsen, P.H.; Van Den Heuvel, O.A.; Veale, D.; et al. Impulse control disorders and “behavioural addictions” in the ICD-11. World Psychiatry 2014, 13, 125–127. [Google Scholar] [CrossRef]
- Dickenson, J.A.; Gleason, N.; Coleman, E.; Miner, M.H. Prevalence of Distress Associated With Difficulty Controlling Sexual Urges, Feelings, and Behaviors in the United States. JAMA Netw. Open 2018, 1, e184468. [Google Scholar] [CrossRef]
- Reid, R.C.; Carpenter, B.N.; Hook, J.N.; Garos, S.; Manning, J.C.; Gilliland, R.; Cooper, E.B.; Mckittrick, H.; Davtian, M.; Fong, T. Report of findings in a dsm-5 field trial for hypersexual disorder. J. Sex. Med. 2012, 9, 2868–2877. [Google Scholar] [CrossRef]
- Cooper, A. Sexuality and the Internet: Surfing into the New Millennium. CyberPsychology Behav. 1998, 1, 187–193. [Google Scholar] [CrossRef]
- Cooper, A.; Delmonico, D.L.; Burg, R. Cybersex users, abusers, and compulsives: New findings and implications. Sex. Addict. Compulsivity J. Treat. Prev. 2000, 7, 5–29. [Google Scholar] [CrossRef]
- Döring, N.M. The Internet’s impact on sexuality: A critical review of 15 years of research. Comput. Hum. Behav. 2009, 25, 1089–1101. [Google Scholar] [CrossRef]
- Young, K.S. Internet Sex Addiction Risk Factors, Stages of Development, and Treatment. Am. Behav. Sci. 2008, 52, 21–37. [Google Scholar] [CrossRef]
- Wéry, A.; Vogelaere, K.; Challet-Bouju, G.; Poudat, F.-X.; Caillon, J.; Lever, D.; Billieux, J.; Grall-Bronnec, M. Characteristics of self-identified sexual addicts in a behavioral addiction outpatient clinic. J. Behav. Addict. 2016, 5, 623–630. [Google Scholar] [CrossRef]
- Carnes, P.J. Sexual addiction and compulsion: Recognition, treatment & recovery. CNS Spectr. 2000, 5, 63–72. [Google Scholar]
- Carroll, J.S.; Padilla-Walker, L.M.; Nelson, L.J.; Olson, C.D.; Barry, C.M.; Madsen, S.D. Generation XXX: Pornography Acceptance and Use Among Emerging Adults. J. Adolesc. Res. 2008, 23, 6–30. [Google Scholar] [CrossRef]
- Häggström-Nordin, E.; Hanson, U.; Tydén, T. Associations between pornography consumption and sexual practices among adolescents in Sweden. Int. J. STD AIDS 2005, 16, 102–107. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Cain, D. The relationship between indicators of sexual compulsivity and high risk sexual practices among men and women receiving services from a sexually transmitted infection clinic. J. Sex Res. 2004, 41, 235–241. [Google Scholar] [CrossRef]
- Mick, T.M.; Hollander, E. Impulsive-Compulsive Sexual Behavior. CNS Spectr. 2006, 11, 944–955. [Google Scholar] [CrossRef]
- Raymond, N.C.; Coleman, E.; Miner, M.H. Psychiatric comorbidity and compulsive/impulsive traits in compulsive sexual behavior. Compr. Psychiatry 2003, 44, 370–380. [Google Scholar] [CrossRef]
- de Tubino Scanavino, M.; Ventuneac, A.; Abdo, C.H.N.; Tavares, H.; do Amaral, M.L.S.A.; Messina, B.; dos Reis, S.C.; Martins, J.P.L.B.; Parsons, J.T. Compulsive sexual behavior and psychopathology among treatment-seeking men in São Paulo, Brazil. Psychiatry Res. 2013, 209, 518–524. [Google Scholar] [CrossRef]
- Carnes Don’t Call It Love; Bantam Books: New York, NY, USA, 1991; ISBN 0-553-35138-9.
- Reid, R.C.; Cyders, M.A.; Moghaddam, J.F.; Fong, T.W. Psychometric properties of the Barratt Impulsiveness Scale in patients with gambling disorders, hypersexuality, and methamphetamine dependence. Addict. Behav. 2014, 39, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.C.; Dhuffar, M.K.; Parhami, I.; Fong, T.W. Exploring facets of personality in a patient sample of hypersexual women compared with hypersexual men. J. Psychiatry Pract. 2012, 18, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.C.; Berlin, H.A.; Kingston, D.A. Sexual impulsivity in hypersexual men. Curr. Behav. Neurosci. Rep. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Mechelmans, D.J.; Irvine, M.; Banca, P.; Porter, L.; Mitchell, S.; Mole, T.B.; Lapa, T.R.; Harrison, N.A.; Potenza, M.N.; Voon, V. Enhanced attentional bias towards sexually explicit cues in individuals with and without compulsive sexual behaviours. PLoS ONE 2014, 9, e105476. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.C.; Karim, R.; McCrory, E.; Carpenter, B.N. Self-reported differences on measures of executive function and hypersexual behavior in a patient and community sample of men. Int. J. Neurosci. 2010, 120, 120–127. [Google Scholar] [CrossRef]
- Schiebener, J.; Laier, C.; Brand, M. Getting stuck with pornography? Overuse or neglect of cybersex cues in a multitasking situation is related to symptoms of cybersex addiction. J. Behav. Addict. 2015, 4, 14–21. [Google Scholar] [CrossRef]
- Baumeister, R.F.; Catanese, K.R.; Vohs, K.D. Is there a gender difference in strength of sex drive? Theoretical views, conceptual distinctions, and a review of relevant evidence. Personal. Soc. Psychol. Rev. 2001, 5, 242–273. [Google Scholar] [CrossRef]
- Hönekopp, J.; Bartholdt, L.; Beier, L.; Liebert, A. Second to fourth digit length ratio (2D:4D) and adult sex hormone levels: New data and a meta-analytic review. Psychoneuroendocrinology 2007, 32, 313–321. [Google Scholar] [CrossRef]
- Hönekopp, J.; Voracek, M.; Manning, J.T. 2nd to 4th digit ratio (2D:4D) and number of sex partners: Evidence for effects of prenatal testosterone in men. Psychoneuroendocrinology 2006, 31, 30–37. [Google Scholar] [CrossRef]
- Klimek, M.; Andrzej, G.; Nenko, I.; Alvarado, L.C.; Jasienska, G. Digit ratio (2D:4D) as an indicator of body size, testosterone concentration and number of children in human males. Ann. Hum. Biol. 2014, 41, 518–523. [Google Scholar] [CrossRef]
- Varella, M.A.C.; Valentova, J.V.; Pereira, K.J.; Bussab, V.S.R. Promiscuity is related to masculine and feminine body traits in both men and women: Evidence from Brazilian and Czech samples. Behav. Processes 2014, 109, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Katehakis, A. Affective Neuroscience and the Treatment of Sexual Addiction. Sex. Addict. Compulsivity 2009, 16, 1–31. [Google Scholar] [CrossRef]
- Walton, M.T.; Bhullar, N. Compulsive Sexual Behavior as an Impulse Control Disorder: Awaiting Field Studies Data. Arch. Sex. Behav. 2018, 47, 1327–1831. [Google Scholar] [CrossRef]
- Reid, R.C.; Garos, S.; Carpenter, B.N. Reliability, validity, and psychometric development of the hypersexual behavior inventory in an outpatient sample of men. Sex. Addict. Compulsivity 2011, 18, 30–51. [Google Scholar] [CrossRef]
- Bernstein, D.; Fink, L. Manual for the Childhood Trauma Questionnaire (CTQ); The Psychological Corporation: New York, NY, USA, 1998. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-IV Wechsler Adult Intelligence Scale Deutschsprachige Adaption, 4th ed.; Petermann, F., Petermann, U., Eds.; Hogrefe: Göttingen, Germany, 2013. [Google Scholar]
- Janssen, E.; Vorst, H.; Finn, P.; Bancroft, J. The sexual inhibition (SIS) and sexual excitation (SES) scales: I. Measuring sexual inhibition and excitation proneness in men. J. Sex Res. 2002, 39, 114–126. [Google Scholar] [CrossRef]
- Pawlikowski, M.; Altstötter-Gleich, C.; Brand, M. Validation and psychometric properties of a short version of Young’s Internet Addiction Test. Comput. Hum. Behav. 2013, 29, 1212–1223. [Google Scholar] [CrossRef]
- Carnes, P.; Green, B.; Carnes, S. The same yet different: Refocusing the Sexual Addiction Screening Test (SAST) to reflect orientation and gender. Sex. Addict. Compulsivity 2010, 17, 7–30. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Wunderlich, U.; Gruschwitz, S.; Zaudig, M. SKID I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft und Beurteilungsheft. Eine deutschsprachige, erweiterte Bearb. d. amerikanischen Originalversion des SKID I; Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Barratt Impulsiveness Scale (BIS-11). J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Fagerström, O.K.; Schneider, N.G. Fagerström Test for Nicotine Dependence. J Behav Med. 1989, 12, 159–181. [Google Scholar]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De la Fuente, J.R.; Grant, M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Hautzinger, M.; Keller, F.; Kühner, C. Beck Depressions-Inventar II. Deutsche Bearbeitung und Handbuch zum BDI II.; Harcourt Test Services: Frankfurt am Main, Germany, 2006. [Google Scholar]
- Fraley, R.C.; Waller, N.G.; Brennan, K.A. An item response theory analysis of self-report measures of adult attachment. J. Pers. Soc. Psychol. 2000, 78, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, J.; Brosig, B.; Brähler, E. TAS-26: Toronto-Alexithymie-Skala-26 (deutsche Version); Hogrefe: Göttingen, Germany, 2001. [Google Scholar]
- Gross, J.J.; John, O.P. Individual Differences in Two Emotion Regulation Processes: Implications for Affect, Relationships, and Well-Being. J. Pers. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Petermann, F. Fragebogen zur Erhebung der Emotionsregulation bei Erwachsenen (FEEL-E). Zeitschrift fur Psychiatry Psychol. Psychother. 2015, 63, 67–68. [Google Scholar] [CrossRef]
- Retz-Junginger, P.; Retz, W.; Blocher, D.; Weijers, H.-G.; Trott, G.-E.; Wender, P.H.; Rössler, M. Wender Utah Rating Scale (WURS-k) Die deutsche Kurzform zur retrospektiven erfassung des hyperkinetischen syndroms bei erwachsenen. Nervenarzt 2002, 73, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Rösler, M.; Retz, W.; Retz-Junginger, P.; Thome, J.; Supprian, T.; Nissen, T.; Stieglitz, R.D.; Blocher, D.; Hengesch, G.; Trott, G.E. Instrumente zur Diagnostik der Aufmerksamkeitsdefizit-/Hyperaktivitätsstörung (ADHS) im Erwachsenenalter. Nervenarzt 2004, 75, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Agresti, A. An Introduction to Categorical Data Analysis, 2nd ed.; Wiley: Hoboken, NJ, USA, 2018; ISBN 1119405262. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 9780805802832. [Google Scholar]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B. Structured Clinical Interview for DSM-IV Axis I Disorder; New York State Psychiatric Institute: New York, NY, USA, 1995. [Google Scholar]
- Carvalho Fernando, S.; Beblo, T.; Schlosser, N.; Terfehr, K.; Otte, C.; Löwe, B.; Wolf, O.T.; Spitzer, C.; Driessen, M.; Wingenfeld, K. The Impact of Self-Reported Childhood Trauma on Emotion Regulation in Borderline Personality Disorder and Major Depression. J. Trauma Dissociation 2014, 15, 384–401. [Google Scholar] [CrossRef]
- Goodman, S.H.; Gotlib, I.H. Risk for Psychopathology in the Children of Depressed Mothers: A Developmental Model for Understanding Mechanisms of Transmission. Psychol. Rev. 1999, 106, 458–490. [Google Scholar] [CrossRef]
- Waters, S.F.; Virmani, E.A.; Thompson, R.A.; Meyer, S.; Raikes, H.A.; Jochem, R. Emotion regulation and attachment: Unpacking two constructs and their association. J. Psychopathol. Behav. Assess. 2010, 32, 37–47. [Google Scholar] [CrossRef]
- Beutel, M.E.; Giralt, S.; Wölfling, K.; Stöbel-Richter, Y.; Subic-Wrana, C.; Reiner, I.; Tibubos, A.N.; Brähler, E. Prevalence and determinants of online-sex use in the German population. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Reid, R.C.; Carpenter, B.N.; Spackman, M.; Willes, D.L. Alexithymia, emotional instability, and vulnerability to stress proneness in patients seeking help for hypersexual behavior. J. Sex Marital Ther. 2008, 34, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Voon, V.; Mole, T.B.; Banca, P.; Porter, L.; Morris, L.; Mitchell, S.; Lapa, T.R.; Karr, J.; Harrison, N.A.; Potenza, M.N.; et al. Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLoS ONE 2014, 9, e102419. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.D.; Paglia, H.A.; Redden, S.A.; Grant, J.E. Age at first sexual activity: Clinical and cognitive associations. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatry 2018, 30, 102–112. [Google Scholar]
- Gola, M.; Lewczuk, K.; Skorko, M. What matters: Quantity or quality of pornography use? Psychological and behavioral factors of seeking treatment for problematic pornography use. J. Sex. Med. 2016, 13, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 2008, 199, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Rettenberger, M.; Klein, V.; Briken, P. The Relationship Between Hypersexual Behavior, Sexual Excitation, Sexual Inhibition, and Personality Traits. Arch. Sex. Behav. 2016, 45, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Klein, V.; Schmidt, A.F.; Turner, D.; Briken, P. Are sex drive and hypersexuality associated with pedophilic interest and child sexual abuse in a male community sample? PLoS ONE 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Mann, R.E.; Hanson, R.K.; Thornton, D. Assessing risk for sexual recidivism: Some proposals on the nature of psychologically meaningful risk factors. Sex. Abuse J. Res. Treat. 2010, 22, 191–217. [Google Scholar] [CrossRef]
- Kafka, M.P.; Hennen, J. A DSM-IV Axis I Comorbidity Study of Males (n = 120) With Paraphilias and Paraphilia-Related Disorders. Sex. Abuse 2002, 14, 349–366. [Google Scholar] [CrossRef]
- Weiss, D. The prevalence of depression in male sex addicts residing in the United States. Sex. Addict. Compulsivity 2004, 11, 57–69. [Google Scholar] [CrossRef]
- Hagedorn, W.B. The call for a new Diagnostic and Statistical Manual of Mental Disorders diagnosis: Addictive disorders. J. Addict. Offender Couns. 2009, 29, 110–127. [Google Scholar] [CrossRef]
- Kaplan, M.S.; Krueger, R.B. Diagnosis, assessment, and treatment of hypersexuality. J. Sex Res. 2010, 47, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Maclean, J.C.; Xu, H.; French, M.T.; Ettner, S.L. Mental health and risky sexual behaviors: Evidence from DSM-IV Axis II disorders. J. Ment. Health Policy Econ. 2013, 16, 187–208. [Google Scholar] [PubMed]
- Reid, R.C.; Davtian, M.; Lenartowicz, A.; Torrevillas, R.M.; Fong, T.W. Perspectives on the assessment and treatment of adult ADHD in hypersexual men. Neuropsychiatry 2013, 3, 295–308. [Google Scholar] [CrossRef]
- Hallberg, J.; Kaldo, V.; Arver, S.; Dhejne, C.; Öberg, K.G. A cognitive-behavioral therapy group intervention for hypersexual disorder: A feasibility study. J. Sex. Med. 2017, 14, 950–958. [Google Scholar] [CrossRef] [PubMed]
