Link to abstract – J Adolesc. 2019 Feb 9;72:10-13. doi: 10.1016/j.adolescence.2019.01.006.
Abstract
INTRODUCTION: The focus of this brief literature review is to explore whether there is a relationship between the unique anatomical and physiological paradigms of the adolescent brain and an increased sensitivity to sexually explicit material.
METHODS: The EBSCO Research Data bases were searched using the following key terms: adolescence, adolescent brain development, neuroplasticity, sexually explicit material, sexualization, and pornography.
RESULTS: The literature highlighted several components of the adolescent brain that are different than the mature brain. These include: an immature prefrontal cortex and over-responsive limbic and striatal circuits, heightened period for neuroplasticity, overactive dopamine system, a pronounced HPA axis, augmented levels of testosterone, and the unique impact of steroid hormones. The physiological response to sexually explicit material is delineated. The overlap of key areas associated with the unique adolescent brain development and sexually explicit material is noteworthy. A working model summary that compares the response of the adult and adolescent brain to the same sexually explicit stimulus is outlined.
CONCLUSIONS: The literature suggests that the adolescent brain may indeed be more sensitive to sexually explicit material, but due to a lack of empirical studies this question cannot be answered definitively. Suggestions for future research are given to further advance the work in this applicable field of today.
KEYWORDS: Adolescence; Adolescent brain development; Neuroplasticity; Pornography; Sexualization; Sexually explicit material
PMID: 30754014
DOI: 10.1016/j.adolescence.2019.01.006
Unique paradigms of the adolescent brain
The focus of this brief literature review is to explore whether there is a relationship between the unique anatomical and physiological paradigms of the adolescent brain and an increased sensitivity to sexually explicit material. The EBSCO Research Data bases were searched using the following key terms: adolescence, adolescent brain development, neuroplasticity, sexually explicit material, sexualization, pornography. Adolescence is the period between childhood and adulthood encompassed by changes in physical, psychological, and social development (Ernst, Pine, & Hardin, 2006).
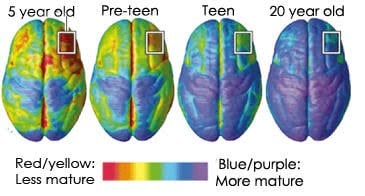
The unique paradigms of the adolescent brain include the following: 1) An immature prefrontal cortex and over-responsive limbic and striatal circuits (Dumontheil, 2016; Somerville & Jones, 2010; Somerville, Hare, & Casey, 2011; Van Leijenhorst et al., 2010; Vigil et al., 2011); 2) A heightened period for neuroplasticity (McCormick & Mathews, 2007; Schulz & Sisk, 2006; Sisk & Zehr, 2005; Vigil et al., 2011); 3) Overactive dopamine system (Andersen, Rutstein, Benzo, Hostetter, & Teicher, 1997; Ernst et al., 2005; Luciana, Wahlstrom, & White, 2010; Somerville & Jones, 2010; Wahlstrom, White, & Luciana, 2010); 4) A pronounced HPA axis (Dahl & Gunnar, 2009; McCormick & Mathews, 2007; Romeo, Lee, Chhua, McPherson, & McEwan, 2004; Walker, Sabuwalla, & Huot, 2004); 5) Augmented levels of testosterone (Dorn et al., 2003; Vogel, 2008; Mayo Clinic/Mayo Medical Laboratories, 2017); and 6) The unique impact of steroid hormones (cortisol and testosterone) on brain development during the organizational window of adolescence (Brown & Spencer, 2013; Peper, Hulshoff Pol, Crone, Van Honk, 2011; Sisk & Zehr, 2005; Vigil et al., 2011).
Blakemore and colleagues have led the field in adolescent brain development and has opined that the teenage years should be considered a sensitive period due to the dramatic brain reorganization that is taking place (Blakemore, 2012). The areas of the brain that undergo the most change during adolescence include internal control, multi-tasking and planning (Blakemore, 2012).
Blakemore and Robbins (2012) linked adolescence to risky decision making and attributed this characteristic to the dissociation between the relatively slow, linear development of impulse control and response inhibition during adolescence versus the nonlinear development of the reward system, which is often hyper-responsive to rewards in adolescence.
Sexually explicit material
Sexually explicit material activates the amygdala of the limbic system (Ferretti et al., 2005; Karama et al., 2002; Redoute et al., 2000; Walter et al., 2008). Activation of the amygdala concomitantly initiates the following: 1) the hypothalamus activates neurons in the brain stem and spinal cord initiating the sympathetic division of the autonomic nervous system resulting in systemic release of epinephrine and norepinephrine; 2) the hypothalamus stimulates the pituitary gland, resulting in cortisol release via the hypothalamic– pituitary–adrenal (HPA) axis, and testosterone release via the hypothalamic–pituitary–gonadal (HPG) axis (Viau, 2002); 3) the nucleus accumbens is activated via dopamine. For a comprehensive review of the amygdala and its innervations and regulation of somatic processes see Mirolli, Mannella, and Baldassarre (2010). The function of the prefrontal cortex is decreased, and the function of the basal ganglia is increased due to the release of neurotransmitters (Arnsten, 2009; Hanson et al., 2012; Radley, 2005).
Both infrequent and frequent use of pornographic internet sites were significantly associated with social maladjustment among Greek adolescents (Tsitsika et al., 2009). Pornography use contributed to delay discounting, or an individual’s tendency to discount future outcomes in favor of immediate rewards (Negash, Sheppard, Lambert, & Fincham, 2016). Negash and colleagues used a sample that had an average age of 19 and 20, which the author highlighted were still biologically considered adolescents. They reiterated that their samples did not report being addictive or compulsive users, but alterations in decision making processes were still shown.
Pornography use is linked to stimulus and neuroplasticity of the mesolimbic dopaminergic reward system (Hilton, 2013). MRI scans found a significant negative association between reported pornography hours per week and gray matter volume in the right caudate and functional connectivity with the dorsolateral prefrontal cortex (Kuhn & Gallinat, 2014). Pornography could be the cause of this neuroplasticity, but a precondition that makes pornography consumption more rewarding could not be ruled out.
Working model summary
We propose a working model summary, considering the unique paradigms of the adolescent brain and the characteristics of sexually explicit material. The overlap of key areas associated with the unique adolescent brain and sexually explicit material is noteworthy.
Upon exposure to sexually explicit material, the stimulation of the amygdala and the HPA axis would be enhanced in the adolescent, compared with the adult. This would lead to a more pronounced curtailment of the prefrontal cortex and enhanced activation of the basal ganglia in the adolescent. This condition, therefore, would compromise executive function, which includes inhibition and self-control, and enhances impulsivity. Because the adolescent’s brain is still developing, it is more conducive to neuroplasticity. The prefrontal cortex going “off-line,” so to speak, drives the subtle rewiring that favors subcortical development. If the neuroplasticity imbalance continues over time, this may result in a relatively weakened cortical circuit in favor of a more dominant subcortical circuit, which could predispose the adolescent to continued self-gratification and impulsivity. The adolescent’s nucleus accumbens, or pleasure center of the brain, would have an exaggerated stimulation compared to the adult. The increased levels of dopamine would translate into augmented emotions associated with dopamine, such as pleasure and craving (Berridge, 2006; Volkow, 2006).
Due to the pubertal surge of testosterone, its level would also be augmented in comparison to the adult. This increase in testosterone may lead to higher tendencies of aggression (Banks & Dabbs, 1996; Goetz et al., 2014; Nelson, Leibenluft, McClure, & Pine, 2005; Schulz & Sisk, 2006) and sexual anticipation (Amstislavskaya & Popova, 2004; Bonilla–Jaime, Vazquez-Palacios, Arteaga-Silva, & Retana-Marquez, 2006; Exton et al., 1999; Redoute et al., 2000; Stoleru et al., 1999; ).
Because of the organizational window of development during adolescence, cortisol and testosterone would have a unique affect upon brain organization or the inherent viability of various neural circuits. This effect would not be found in the adult because this specific window of organization has closed. Chronic exposure to cortisol has the potential, during the adolescent organizational period, to drive neuroplasticity that results in compromised cognitive function and stress resilience even through adulthood (McEwen, 2004; Tsoory & Richter-Levin, 2006; Tsoory, 2008; McCormick & Mathews, 2007; 2010). The robustness of the amygdala post puberty, at least in part, depends on the magnitude of testosterone exposure during the critical adolescent developmental window (De Lorme, Schulz, Salas-Ramirez, & Sisk, 2012; De Lorme & Sisk, 2013; Neufang et al., 2009; Sarkey, Azcoitia, Garcia- Segura, Garcia-Ovejero, & DonCarlos, 2008). A robust amygdala is linked to heightened levels of emotionality and compromised self-regulation (Amaral, 2003; Lorberbaum et al., 2004; De Lorme & Sisk, 2013).
Discussion and future direction
This paper sought to begin the academic conversation: Could adolescents be more sensitive to sexually explicit material due to the unique anatomical and physiological paradigms of the adolescent brain? The current literature suggests that the adolescent brain may indeed be more sensitive to sexually explicit material, but due to the lack of empirical studies this question cannot be answered definitively. The challenge of working through ethical considerations for controlled studies is also a significant, albeit understandable, barrier toward scientific progress in this field.
As a start, we recommend conducting population studies using self-assessment surveys that inquire of behavioral tendencies before initial exposure to sexually explicit material, and after different degrees of exposure. Surveys could also be given to parents to ascertain whether the parent-child relationship is a significant factor toward child health self-efficacy (and scholastic performance).
Another research avenue to consider is the role of technology as a gateway for adolescents to be exposed to sexually explicit material. Since actual social media use can be tracked and compared to perceived use, surveys that ask participants to self-evaluate their technology usage and exposure to sexually explicit material would be a fairly straight-forward study to conduct.
Ultimately, a paramount contribution to this field could be a longitudinal study that would involve following a group of children through adolescence and into adulthood concomitant with documented medical history and the acquisition of anatomical, physiological, and psychological data from regularly scheduled structural and functional MRI, and/or PET imaging.
Designing careful, ethical studies to investigate the effect of sexually explicit material exposure on the adolescent brain is a necessary step toward understanding the variability of adult experiences with sexually explicit material.
References
- Amaral, D. G. (2003). The amygdala, social behavior, and danger detection. Annals of the New York Academy of Sciences, 1000, 337–347. https://doi.org/10.1196/
annals.1280.015. - Amstislavskaya, T. G., & Popova, N. K. (2004). Female-induced sexual arousal in male mice and rats: Behavioral and testosterone response. Hormones and Behavior, 46,
544–550. - Andersen, S. L., Rutstein, M., Benzo, J. M., Hostetter, J. C., & Teicher, M. H. (1997). Sex differences in dopamine receptor overproduction and elimination. NeuroReport,
8, 1495–1498. https://doi.org/10.1097/00001756-199704140-00034. - Arnsten, A. F. T. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10(6), 410–422. https://doi.org/
10.1038/nrn2648. - Banks, T., & Dabbs, J. M., Jr. (1996). Salivary testosterone and cortisol in a delinquent and violent urban subculture. The Journal of Social Psychology, 136(1), 49–56.
https://doi.org/10.1080/00224545.1996.9923028. - Berridge, K. C. (2006). The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology, 191, 391–431. https://doi.org/10.1007/
s00213-006-0578-x. - Blakemore, S. (2012). Development of the social brain in adolescence. Journal of the Royal Society of Medicine, 105, 111–116. https://doi.org/10.1258/jrsm.2011.
110221. - Blakemore, S., & Robbins, T. W. (2012). Decision-making in the adolescent brain. Nature Neuroscience, 15(9), 1184–1191. https://doi.org/10.1038/nn.3177.
- Bonilla-Jaime, H., Vazquez-Palacios, G., Arteaga-Silva, M., & Retana-Marquez, S. (2006). Hormonal responses to different sexually related conditions in male rats.
Hormones and Behavior, 49, 376–382. - Brown, G. R., & Spencer, K. A. (2013). Steroid hormones, stress and the adolescent brain: A comparative perspective. Neuroscience, 249, 115–128. https://doi.org/10.
1016/j.neuroscience.2012.12.016. - Dahl, R. E., & Gunnar, M. R. (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology.
Development and Psychopathology, 21, 1–6. https://doi.org/10.1017/S0954579409000017. - De Lorme, K. C., Schulz, K. M., Salas-Ramirez, K. Y., & Sisk, C. L. (2012). Pubertal testosterone organizes regional volume and neuronal number within the medial
amygdala of adult male Syrian hamsters. Brain Research, 1460, 33–40. https://doi.org/10.1016/j.brainres.2012.04.035. - De Lorme, K. C., & Sisk, C. L. (2013). Pubertal testosterone programs context-appropriate agonistic behavior and associated neural activation patterns in male Syrian
hamsters. Psychology & Behavior, 112–113, 1–7. https://doi.org/10.1016/j.physbeh.2013.02.003. - Dorn, L. D., Dahl, R. E., Williamson, D. E., Birmaher, B., Axelson, D., Perel, J., et al. (2003). Developmental markers in adolescence: Implications for studies of pubertal
processes. Journal of Youth and Adolescence, 32(5), 315–324. - Dumontheil, I. (2016). Adolescent brain development. Current Opinion in Behavioral Sciences, 10, 39–44. https://doi.org/10.1016/j.cobeha.2016.04.012.
- Ernst, M., Nelson, E. E., Jazbec, S., McClure, E. B., Monk, C. S., Leibenluft, E., et al. (2005). Amygdala and nucleus accumbens in responses to receipt and omission of
gains in adults and adolescents. NeuroImage, 25, 1279–1291. https://doi.org/10.1016/j.neuroimage.2004.12.038. - Ernst, M., Pine, D. S., & Hardin, M. (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36(3), 299–312.
- Exton, M. S., Bindert, A., Kruger, T., Scheller, F., Hartmann, U., & Schedlowski, M. (1999). Cardiovascular and endocrine alterations after masturbation-induced
orgasm in women. Psychosomatic Medicine, 61, 280–289. - Ferretti, A., Caulo, M., Del Gratta, C., Di Matteo, R., Merla, A., Montorsi, F., et al. (2005). Dynamics of male sexual arousal: Distinct components of brain activation
revealed by fMRI. NeuroImage, 26, 1086–1096. https://doi.org/10.1016/j.neuromiage.2005.03.025. - Goetz, S. M. M., Tang, L., Thomason, M. E., Diamond, M. P., Hariri, A. R., & Carre, J. M. (2014). Testosterone rapidly increases neural reactivity to threat in healthy
men: A novel two-step pharmacological challenge paradigm. Biological Psychiatry, 76, 324–331. - Hanson, J. L., Chung, M. K., Avants, B. B., Rudolph, K. D., Shirtcliff, E. A., Gee, J. C., et al. (2012). Structural variations in prefrontal cortex mediate the relationship
between early childhood stress and spatial working memory. The Journal of Neuroscience, 32(23), 7917–7925. https://doi.org/10.1523/jneurosci.0307-12.2012. - Hilton, D. L. (2013). Pornography addiction – a supranormal stimulus considered in the context of neuroplasticity. Socioaffective Neuroscience & Psychology, 3, 20767.
https://doi.org/10.3402/snp.v3i0.20767. - Karama, S., Lecours, A. R., Leroux, J., Bourgouin, P., Beaudoin, G., Joubert, S., et al. (2002). Areas of brain activation in males and females during viewing of erotic
film excerpts. Human Brain Mapping, 16, 1–13. https://doi.org/10.1002/hbm.10014. - Kuhn, S., & Gallinat, J. (2014). Brain structure and functional connectivity associated with pornography consumption. JAMA Psychiatry. https://doi.org/10.1001/
jamapsychiatry.2014.93. - Lorberbaum, J. P., Kose, S., Johnson, M. R., Arana, G. W., Sullivan, L. K., Hamner, M. B., et al. (2004). Neural correlates of speech anticipatory anxiety in generalized
social phobia. NeuroReport, 15(18), 2701–2705. - Luciana, M., Wahlstrom, D., & White, T. (2010). Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience & Biobehavioral
Reviews, 34(5), 631–648. https://doi.org/10.1016/j.neubiorev.2009.12.007. - Mayo Clinic (2017). Mayo medical laboratories. Test ID: TTFB testosterone, total, bioavailable, and free, serum. Retrieved from http://www.mayomedicallaboratories.com/
test-catalog/Clinical+and+Interpretive/83686. - McCormick, C. M., & Mathews, I. Z. (2007). HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology, Biochemistry and Behavior, 86, 220–233. https://doi.org/10.1016/j.pbb.2006.07.012.
- McCormick, C.,M., & Mathews, I. Z. (2010). Adolescent development, hypothalamic-pituitary-adrenal function, and programming of adult learning and memory.
- Progress in Neuro-psychopharmacology & Biological Psychiatry, 34, 756–765. https://doi.org/10.1016/j.pnpbp.2009.09.019.
- McEwen, B. (2004). Protection and damage from acute and chronic stress. Annals of the New York Academy of Sciences, 1032, 1–7. https://doi.org/10.1196/annals.
1314.001. - Mirolli, M., Mannella, F., & Baldassarre, G. (2010). The roles of the amygdala in the affective regulation of body, brain, and behavior. Connection Science, 22, 215–245.
https://doi.org/10.1080/09540091003682553. - Negash, S., Sheppard, N., Lambert, N. M., & Fincham, F. D. (2016). Trading later rewards for current pleasure: Pornography consumption and delay discounting. The
Journal of Sex Research, 53(6), 689–700. https://doi.org/10.1080/00224499.2015.1025123. - Nelson, E. E., Leibenluft, E., McClure, E. B., & Pine, D. S. (2005). The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to
psychopathology. Psychological Medicine, 35, 163–174. https://doi.org/10.1017/S0033291704003915. - Neufang, S., Specht, K., Hausmann, M., Gunturkun, O., Herpertz-Dahlmann, B., Fink, G. R., et al. (2009). Sex differences and the impact of steroid hormones on the
developing human brain. Cerebral Cortex, 19, 464–473. https://doi.org/10.1093/cercor/bhn100. - Peper, J. S., Hulshoff Pol, H. E., Crone, E. A., & Van Honk, J. (2011). Sex steroids and brain structure in pubertal boys and girls: A mini-review of neuroimaging studies.
Neuroscience, 191, 28–37. - Radley, J. (2005). Repeated stress and structural plasticity in the brain. Ageing Research Reviews, 4, 271–287. https://doi.org/10.1016/j.arr.2005.03.004.
- Redoute, J., Stoleru, S., Gregoire, M., Costes, N., Cinotti, L., Lavenne, F., et al. (2000). Brain processing of visual sexual stimuli in human males. Human Brain Mapping,
11, 162–177. - Romeo, R. D., Lee, S. J., Chhua, N., McPherson, C. R., & McEwen, B. S. (2004). Testosterone cannot activate an adult-like stress response in prepubertal male rats.
Neuroendocrinology, 79, 125–132. https://doi.org/10.1159/000077270. - Sarkey, S., Azcoitia, I., Garcia-Segura, L. M., Garcia-Ovejero, D., & DonCarlos, L. L. (2008). Classical androgen receptors in non-classical sites in the brain. Hormones
and Behavior, 53, 753–764. - Schulz, K. M., & Sisk, C. L. (2006). Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and
Cellular Endocrinology, 254–256, 120–126. https://doi.org/10.1016/j.mce.2006.04.025. - Sisk, C. L., & Zehr, J. L. (2005). Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26, 163–174. https://doi.org/10.1016/
j.yfrne.2005.10.003. - Somerville, L. H., Hare, T., & Casey, B. J. (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive
Neuroscience, 23, 2123–2134. https://doi.org/10.1162/jocn.2010.21572. - Somerville, L. H., & Jones, R. (2010). Time of change; behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain
and Cognition, 72(1), 124–133. https://doi.org/10.1016/j.bandc.2009.07.003. - Stoleru, S., Gregoire, M. C., Gerard, D., Decety, J., Lafarge, E., Cinotti, L., et al. (1999). Neuroanatomical correlates of visually evoked sexual arousal in human males.
Archives of Sexual Behavior, 28, 1–21. - Tsitsika, A., Critselis, E., Kormas, G., Konstantoulaki, E., Constantopoulos, A., & Kafetzis, D. (2009). Adolescent pornographic internet site use: A multivariate
regression analysis of the predictive factors of use and psychosocial implications. CyberPsychology and Behavior, 12(5), 545–550. https://doi.org/10.1089/cpb.
2008.0346. - Tsoory, M. (2008). Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potential relevance for
mood and anxiety disorders. Neuropsychopharmacology, 33, 378–393. https://doi.org/10.1038/sj.npp.1301397. - Tsoory, M., & Richter-Levin, G. (2006). Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. International Journal of
Neuropsychopharmacology, 9(6), 713–728. https://doi.org/10.1017/S1461145705006255. - Van Leijenhorst, L., Zanolie, K., Van Meel, C. S., Westenberg, P. M., Rombouts, S. A. R. B., & Crone, E. A. (2010). What motivates the adolescent? Brain regions
mediating reward sensitivity across adolescence. Cerebral Cortex, 20, 61–69. https://doi.org/10.1093/cercor/bhp078. - Viau, V. (2002). Functional cross-talk between the hypothalamic-pituitary-gonadal and adrenal axes. Journal of Neuroendocrinology, 14, 506–513.
- Vigil, P., Orellana, R. F., Cortes, M. E., Molina, C. T., Switzer, B. E., & Klaus, H. (2011). Endocrine modulation of the adolescent brain: A review. Journal of Pediatric and
Adolescent Gynecology, 24(6), 330–337. https://doi.org/10.1016/j.jpag.2011.01.061. - Vogel, G. (2008). Time to grow up. Science Now, 2008(863), 1.
- Volkow, N. (2006). Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. The Journal of Neuroscience, 26(24), 6583–6588.
https://doi.org/10.1523/JNEUROSCI.1544-06.2006. - Wahlstrom, D., White, T., & Luciana, M. (2010). Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience & Biobehavioral
Reviews, 34, 631–648. https://doi.org/10.1016/j.neubiorev.2009.12.007. - Walker, E. F., Sabuwalla, Z., & Huot, R. (2004). Pubertal neuromaturation, stress sensitivity, and psychopathology. Development and Psychopathology, 16, 807–824.
https://doi.org/10.1017/S0954579404040027. - Walter, M., Bermpohl, F., Mouras, H., Schiltz, K., Tempelmann, C., Rotte, M., et al. (2008). Distinguishing specific sexual and general emotional effects in fMRI—subcortical and cortical arousal during erotic picture viewing. NeuroImage, 40, 1482–1494. https://doi.org/10.1016/j.neuroimage.2008.01.040.